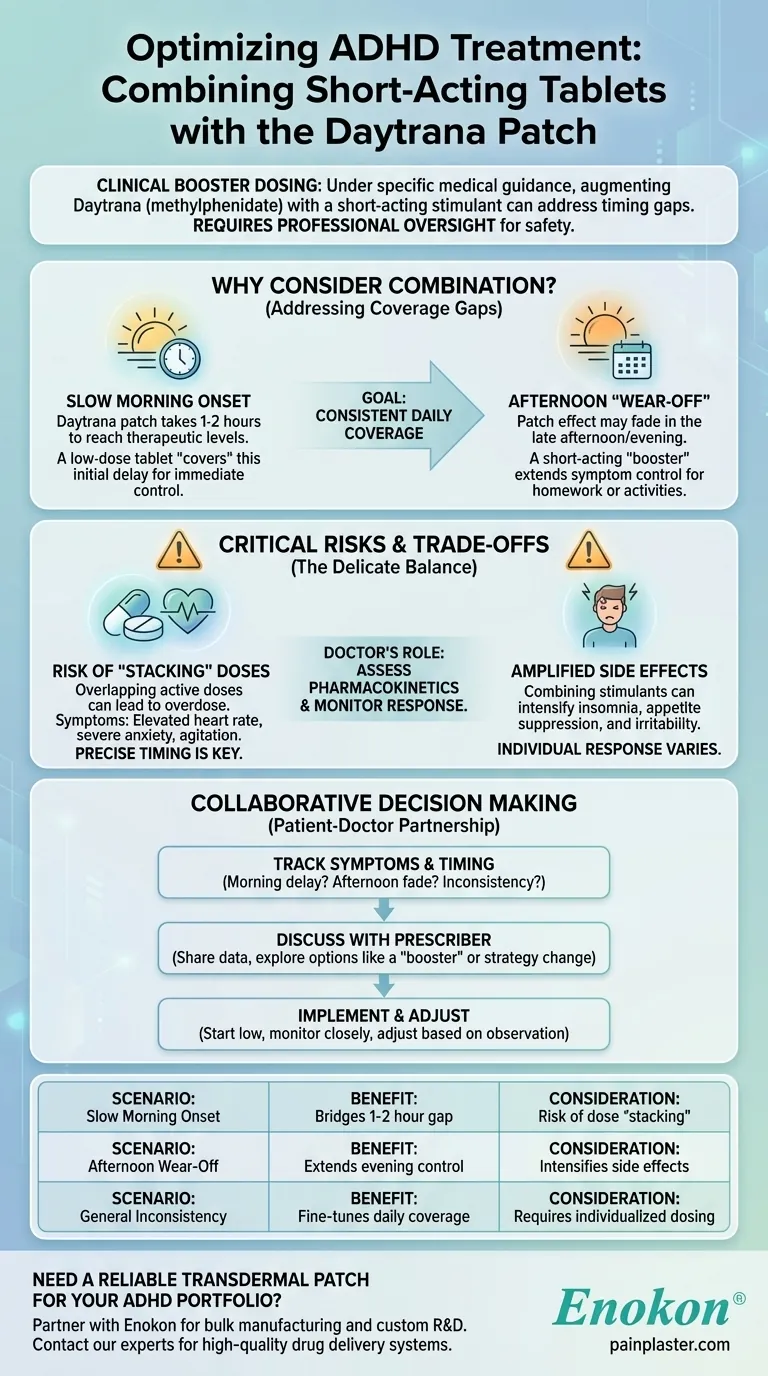
Under specific medical guidance, it is sometimes possible to use a short-acting stimulant tablet in combination with the Daytrana (methylphenidate) patch. This strategy, known as augmentation or "booster dosing," is a clinical decision made by a doctor to fine-tune treatment, often to address a slow onset in the morning or a "wear-off" effect in the afternoon. Attempting this without a doctor's explicit instruction is extremely dangerous.
The core issue is not whether these medications can be combined, but how to do so safely. This requires a deep, professional understanding of their pharmacokinetics—how they are absorbed, peak, and are eliminated by your specific body—to avoid over-medication and manage side effects.

Why a Combination Strategy Might Be Considered
A doctor might consider supplementing the Daytrana patch to solve very specific timing and coverage gaps in ADHD symptom management. The goal is to create a more consistent and effective level of treatment throughout the day.
The Slow Onset Challenge
The Daytrana patch is delivered transdermally (through the skin), which means it can take a significant amount of time, often 1-2 hours, to reach therapeutic levels in the morning.
For individuals who need immediate symptom control upon waking, this delay can be disruptive. A doctor may prescribe a low-dose, short-acting tablet to "cover" this initial gap while the patch begins to work.
The "After-School Slump"
Long-acting medications are designed to last for a specific duration. The Daytrana patch, even when worn for its maximum recommended time, may begin to wear off in the late afternoon or early evening.
This can leave a gap in coverage when focus is still needed for homework, chores, or other activities. A small, short-acting "booster" dose can extend symptom control for a few more hours without the full duration of another long-acting medication.
Understanding the Critical Risks and Trade-offs
Combining stimulants is a delicate balancing act that requires professional oversight. The potential for negative outcomes is significant if not managed correctly.
Risk of "Stacking" Doses
The primary danger is creating an excessive level of medication in the body. Taking an oral stimulant while the patch is still actively delivering its dose can lead to an overdose.
This can result in symptoms like a dangerously elevated heart rate, high blood pressure, severe anxiety, or agitation. A doctor calculates the timing and dosage precisely to avoid this overlap.
Amplified Side Effects
Stimulant medications share common side effects, including appetite suppression, insomnia, irritability, and anxiety.
Combining two forms of the same medication can intensify these side effects. What was a manageable loss of appetite with the patch alone could become severe when a tablet is added, and sleep can be significantly disrupted.
The Importance of Individual Response
Every person metabolizes medication differently. Factors like age, weight, genetics, and even skin type (for the patch) influence the "absorption and effect cycle."
Only a healthcare professional can assess these factors and monitor your response to ensure the combination is both safe and effective. They will start with very low doses and adjust slowly based on careful observation.
Making the Right Choice With Your Doctor
The decision to combine medications is a collaborative one between you and your prescriber. Your role is to provide clear information about when and how your symptoms are, or are not, being managed.
- If your primary concern is the slow start in the morning: Keep a log of when the patch is applied and when you first feel its effects, and ask your doctor if a short-acting form could bridge that gap.
- If your primary concern is coverage for evening activities: Note the specific time you feel the patch's effects begin to fade and discuss whether a planned "booster" dose is a suitable option.
- If you feel the patch's effect is generally inconsistent: Track your symptoms throughout the day over several days to help your doctor determine if the issue is the patch itself or if a different treatment strategy is needed.
Ultimately, effective treatment relies on an open and data-driven partnership with your healthcare provider.
Summary Table:
| Scenario | Potential Benefit | Key Consideration |
|---|---|---|
| Slow Morning Onset | Bridges the 1-2 hour gap before the patch becomes effective. | Risk of dose "stacking" if not timed correctly. |
| Afternoon "Wear-Off" | Extends symptom control for evening activities like homework. | Can intensify side effects like insomnia or appetite loss. |
| General Inconsistency | Fine-tunes daily coverage for more consistent management. | Requires careful, individualized dosing by a doctor. |
Need a Reliable Transdermal Patch for Your ADHD Treatment Portfolio?
Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters. We provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development, ensuring consistent and effective drug delivery systems.
Let us help you develop a high-quality product that meets precise clinical needs. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- How long do side effects from the birth control patch typically last? Navigating the 2-3 Month Adjustment Period
- What role do porous polycarbonate membranes play in controlling the specifications of deformable liposomes? Expert Guide
- What precautions should be taken when using transdermal estradiol? Ensure Safe & Effective Hormone Therapy
- What is the core function of an industrial pressure extruder? Master Liposome Deformability Assessment
- What specific risks are associated with transdermal asenapine use? Key Safety Warnings Explained
- How should estradiol patches be stored and disposed of? A Guide to Safe Handling
- What tissues can microneedle patches target beyond skin? Exploring Versatile Drug Delivery Solutions
- For whom is the birth control patch not as effective? Key Factors for Women Over 198 Lbs or BMI 30+

















