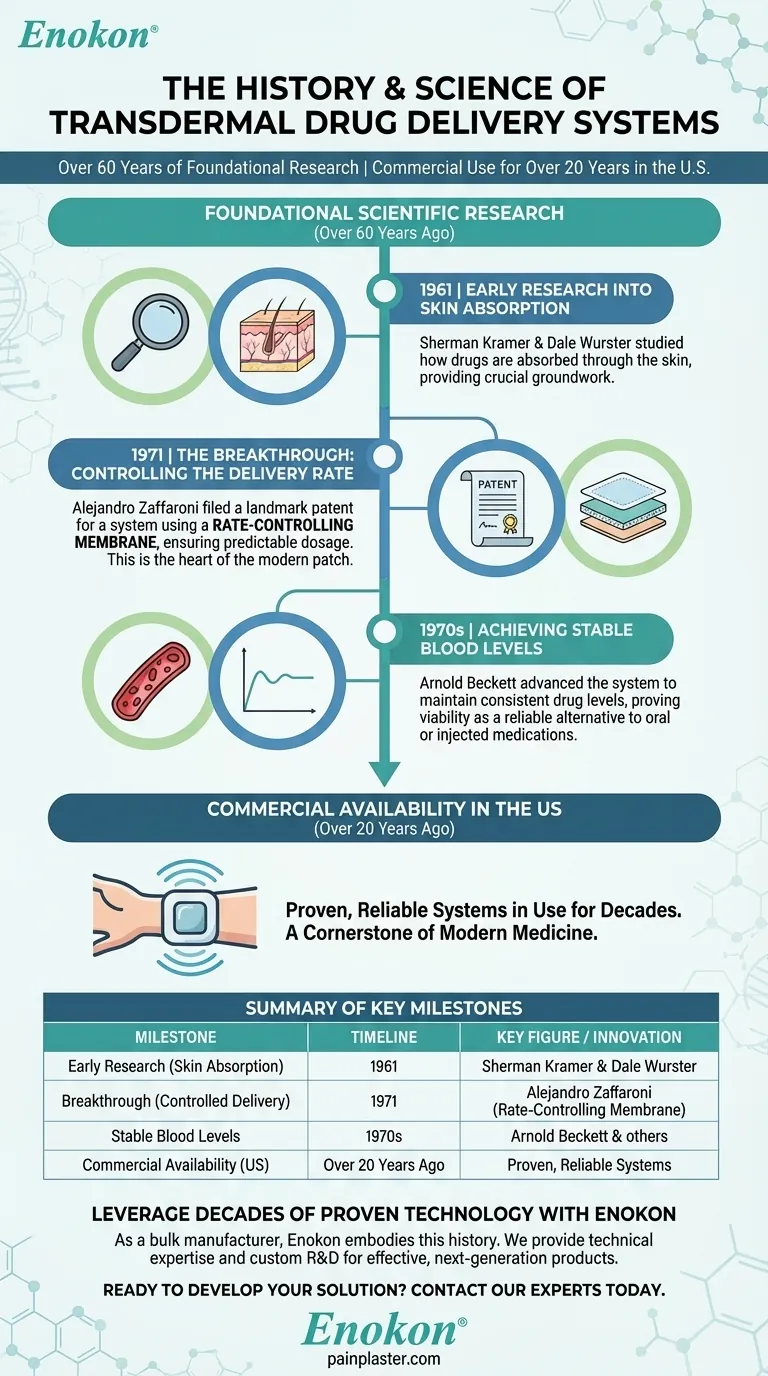
Transdermal drug delivery systems have been a cornerstone of modern medicine for decades. Commercially available in the United States for over 20 years, their scientific foundations trace back even further to critical research and patents from the 1960s and 1970s.
While the patches we see today have been in use for more than two decades, the core technology is not new. It is the result of a long history of scientific inquiry into how substances can be safely and effectively delivered through the skin.

The Scientific Foundations of Transdermal Delivery
To appreciate the maturity of transdermal systems, it's essential to understand the key milestones that made them possible. The journey began long before the first patch was sold.
Early Research into Skin Absorption
The concept was born from early studies in topical drug delivery.
Research in 1961 by Sherman Kramer and Dale Wurster provided crucial groundwork by systematically studying how drugs are absorbed through the skin.
The Breakthrough: Controlling the Delivery Rate
Simple absorption was not enough; the delivery had to be controlled and predictable.
In 1971, Alejandro Zaffaroni filed a landmark patent for a transdermal delivery system that used a rate-controlling membrane, ensuring a steady and predictable dose. This innovation is the conceptual heart of the modern transdermal patch.
Achieving Stable Blood Levels
The final piece of the puzzle was developing a practical system that could maintain consistent drug levels in the bloodstream over time.
Work by Arnold Beckett was instrumental in advancing the patch system to achieve this goal, proving its viability as a reliable alternative to oral or injected medications.
Making the Right Choice for Your Goal
Understanding this history helps frame the technology's place in modern healthcare.
- If your primary focus is the technology's maturity: Recognize that its commercial availability of over 20 years is built upon more than 60 years of foundational scientific research.
- If your primary focus is the core innovation: The key breakthrough was the development of rate-controlling membranes, which transformed simple skin application into a precise drug delivery system.
This long and well-documented history underscores the reliability and established science behind modern transdermal drug delivery.
Summary Table:
| Key Milestone | Timeline | Key Figure/Innovation |
|---|---|---|
| Early Research on Skin Absorption | 1961 | Sherman Kramer & Dale Wurster |
| Breakthrough in Controlled Delivery | 1971 | Alejandro Zaffaroni (Rate-Controlling Membrane) |
| Commercial Availability in the US | Over 20 Years Ago | Proven, reliable systems |
| Foundational Scientific History | Over 60 Years | Arnold Beckett & others |
Leverage Decades of Proven Transdermal Technology with Enokon
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon embodies this long history of innovation. We provide healthcare and pharmaceutical distributors and brands with the technical expertise and custom R&D necessary to develop effective, next-generation transdermal products.
Ready to develop your own reliable transdermal solution? Contact our experts today to discuss your project and benefit from our deep industry knowledge.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Capsaicin Chili Medicated Pain Relief Patches
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits













