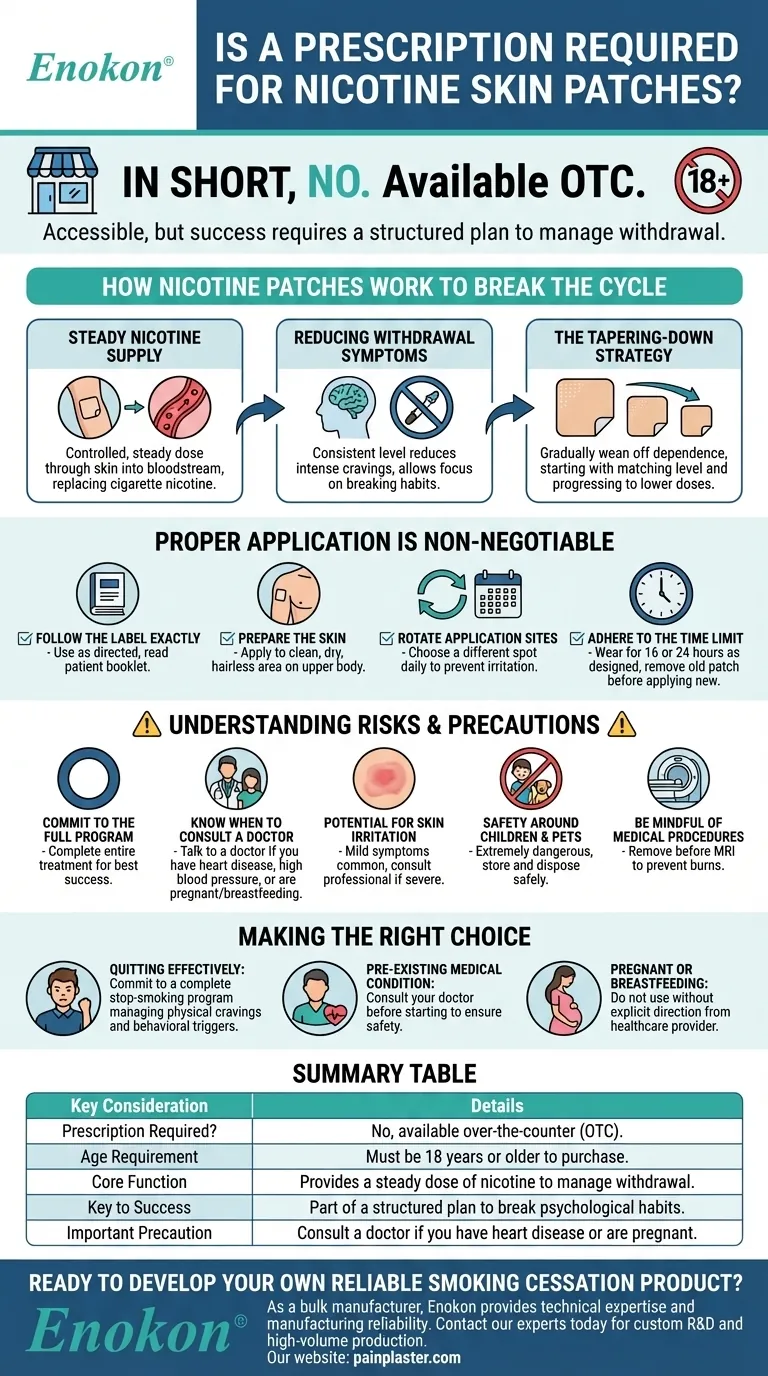
In short, no. A prescription is not required to purchase nicotine skin patches. They are available as an over-the-counter (OTC) product, but you will need to provide proof that you are 18 years of age or older at the time of purchase.
While nicotine patches are accessible without a prescription, their effectiveness is not automatic. Success depends on using them correctly as part of a structured plan to manage withdrawal symptoms and systematically reduce your body's dependence on nicotine.
How Nicotine Patches Work to Break the Cycle
Understanding the mechanism behind nicotine patches is key to using them effectively. They are not a magic cure but a tool designed to ease the transition away from smoking.
A Steady Nicotine Supply
The patch delivers a controlled, steady dose of nicotine directly through your skin and into your bloodstream.
This method is designed to replace the nicotine you would otherwise get from smoking cigarettes.
Reducing Withdrawal Symptoms
By providing a consistent level of nicotine, the patch helps to significantly reduce the intense physical cravings and withdrawal effects that often cause people to relapse.
This stability allows you to focus on breaking the psychological habits associated with smoking.
The Tapering-Down Strategy
The core strategy of patch therapy is to gradually wean your body off its nicotine dependence.
You will start with a patch that matches your current smoking level and, over several weeks, transition to patches with progressively lower doses until you no longer need them.
Proper Application is Non-Negotiable
How you use the patch directly impacts its effectiveness and your comfort. Following the instructions precisely is crucial for success.
Follow the Label Exactly
Always use the patch as directed on the product label or by your doctor. A patient information booklet is often included and should be read carefully.
Prepare the Skin
Apply the patch to a clean, dry, and relatively hairless area of skin on your upper body.
Rotate Application Sites
To prevent skin irritation, choose a different spot to apply the patch each day. Avoid using the same area for at least a week.
Adhere to the Time Limit
Most patches are designed to be worn for either 16 or 24 hours. Remove the old patch before applying a new one.
Understanding the Risks and Precautions
While available OTC, nicotine patches are still a medical treatment with important safety considerations.
Commit to the Full Program
For the best chance of success, you must complete the entire treatment program as outlined. Stopping early can undermine your efforts to quit.
Know When to Consult a Doctor
Before starting, talk to a doctor if you have other medical conditions, especially heart disease, blood vessel disease, or uncontrolled high blood pressure. You should also consult a physician if you are pregnant, breastfeeding, or wish to use the patches longer than the recommended duration.
Potential for Skin Irritation
Mild itching, burning, or redness at the application site is a common side effect. If this becomes severe or doesn't go away, you should consult a healthcare professional.
Safety Around Children and Pets
Both new and used patches contain enough nicotine to be extremely dangerous to children and pets. Always store and dispose of them safely out of reach.
Be Mindful of Medical Procedures
It is critical to remove a nicotine patch before undergoing an MRI scan, as the patch may contain metals that can heat up and cause burns.
Making the Right Choice for Your Quit Journey
Using a nicotine patch correctly increases your chances of quitting successfully. Your specific situation determines the right approach.
- If your primary focus is quitting effectively: Commit to a complete stop-smoking program that uses the patch to manage physical cravings while you address behavioral triggers.
- If you have a pre-existing medical condition (like heart disease): Consult your doctor before starting to ensure the patch is a safe and appropriate choice for you.
- If you are pregnant or breastfeeding: Do not use nicotine patches without explicit direction and supervision from your healthcare provider due to potential risks.
Ultimately, the nicotine patch is an accessible tool, but your success is determined by using it safely and as part of a dedicated plan to quit for good.
Summary Table:
| Key Consideration | Details |
|---|---|
| Prescription Required? | No, available over-the-counter (OTC). |
| Age Requirement | Must be 18 years or older to purchase. |
| Core Function | Provides a steady dose of nicotine to manage withdrawal. |
| Key to Success | Part of a structured plan to break psychological habits. |
| Important Precaution | Consult a doctor if you have heart disease or are pregnant. |
Ready to develop your own reliable smoking cessation product?
As a bulk manufacturer of transdermal patches, Enokon provides the technical expertise and manufacturing reliability that healthcare and pharmaceutical brands need. Whether you require custom R&D for a new nicotine patch formulation or a trusted partner for high-volume production, we ensure quality, consistency, and efficacy.
Let's discuss how we can support your product development goals.
Contact our experts today to explore custom R&D and manufacturing solutions.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Menthol Gel Pain Relief Patch
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
















