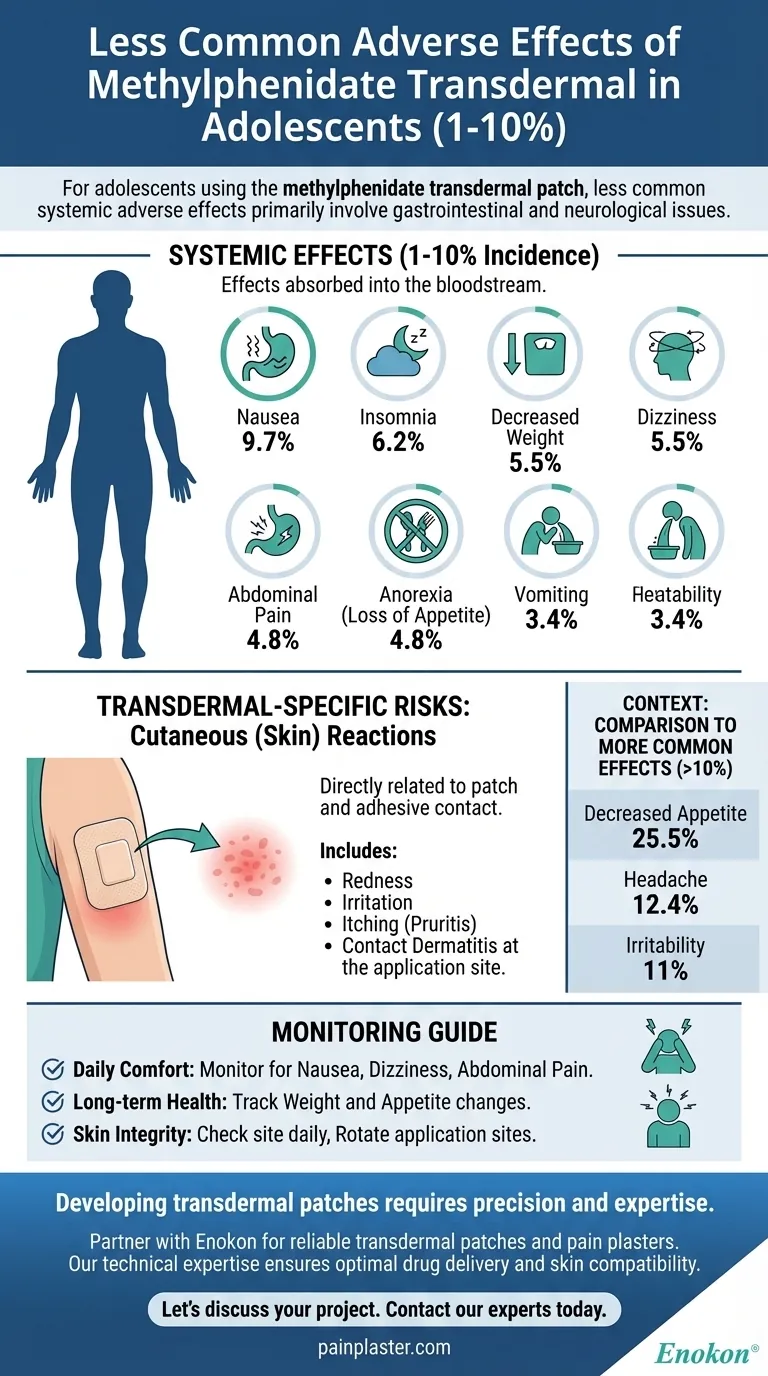
For adolescents using the methylphenidate transdermal patch, the less common systemic adverse effects, occurring in 1% to 10% of users, primarily involve gastrointestinal and neurological issues. These include nausea (9.7%), insomnia (6.2%), decreased weight (5.5%), dizziness (5.5%), abdominal pain (4.8%), anorexia (4.8%), and vomiting (3.4%).
Understanding the full adverse effect profile requires looking beyond these systemic issues. Because this medication is delivered via a skin patch, localized skin reactions are a distinct and common category of side effects that must be monitored separately from the internal effects.

The Two Categories of Adverse Effects
When evaluating the methylphenidate patch, it's essential to separate adverse effects into two distinct groups: systemic effects, which impact the entire body, and cutaneous effects, which are localized to the application site.
Systemic Effects (1-10% Incidence)
These are effects that occur as the medication is absorbed into the bloodstream and circulates throughout the body.
Gastrointestinal Discomfort
Nausea is the most frequent issue in this category, affecting nearly 1 in 10 adolescents.
This is often accompanied by abdominal pain (4.8%) and vomiting (3.4%), creating a cluster of stomach-related side effects.
Appetite and Weight Changes
Anorexia (a loss of appetite) was reported in 4.8% of adolescents, with a resulting decrease in weight observed in 5.5%.
It is critical to note that a more significant decreased appetite is actually one of the most common side effects, occurring in over 25% of adolescents. The weight loss is the less common outcome.
Neurological and Sleep-Related Issues
Insomnia, or difficulty sleeping, is a known stimulant side effect that occurs in 6.2% of adolescent users.
Dizziness was also reported with a similar incidence rate of 5.5%.
Understanding Transdermal-Specific Risks
The delivery method itself—a transdermal patch—introduces a unique set of potential adverse effects that are not seen with oral medications.
Cutaneous (Skin) Reactions
These are common and directly related to the patch and its adhesive components being in contact with the skin.
Reactions can include redness, irritation, itching (pruritis), and various forms of contact dermatitis at the application site.
Distinguishing from Systemic Effects
These skin reactions are localized to where the patch is worn. They are not a sign of an internal or systemic problem but rather a reaction to the patch itself.
Context: How This Compares to More Common Effects
To fully appreciate the "less common" category, it's helpful to compare it against the effects that occur in more than 10% of adolescents.
The Most Common Effects (>10%)
The most frequently reported adverse effects for adolescents are decreased appetite (25.5%), headache (12.4%), and irritability (11%).
This context clarifies why issues like nausea (9.7%) and insomnia (6.2%) are classified in the second tier of frequency.
Monitoring for Adverse Effects: A Practical Guide
Observing and reporting side effects is key to safely managing treatment. Focus your attention based on the potential impact.
- If your primary focus is daily comfort: Monitor for nausea, dizziness, and abdominal pain, as these can directly impact school performance and well-being.
- If your primary focus is long-term health: Regularly track weight and appetite, as sustained changes can have significant health implications over time.
- If your primary focus is skin integrity: Check the skin site daily upon removing the old patch and before applying a new one, and be sure to rotate application sites as directed.
Informed monitoring allows for proactive conversations with a healthcare provider to ensure the treatment remains both effective and well-tolerated.
Summary Table:
| Less Common Systemic Adverse Effect (1-10%) | Incidence Rate in Adolescents |
|---|---|
| Nausea | 9.7% |
| Insomnia | 6.2% |
| Decreased Weight | 5.5% |
| Dizziness | 5.5% |
| Abdominal Pain | 4.8% |
| Anorexia (Loss of Appetite) | 4.8% |
| Vomiting | 3.4% |
Developing a transdermal patch requires precision and expertise to minimize adverse effects.
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon partners with healthcare and pharmaceutical distributors and brands to deliver safe, effective products. Our technical expertise in custom R&D and development ensures optimal drug delivery and skin compatibility, helping you bring higher-quality treatments to market.
Let's discuss your project. Contact our experts today to learn how we can support your transdermal product development.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What serious side effects should be reported immediately when using methylphenidate transdermal? Recognize Critical Warning Signs
- How is a Probe Tack Tester utilized to evaluate the adhesion performance of transdermal films? Ensure Quality & Efficacy
- What is the function of the rate-controlling membrane in a membrane-moderated transdermal patch system? - Stable Delivery
- Does the contraceptive patch affect period pain and related symptoms? Find Relief and Understand the Risks
- What potential cognitive benefits does the estrogen patch offer? Preserve Brain Health with Targeted Delivery
- What is the dosing for Estraderm patch for osteoporosis prevention? Key Guidelines for Effective Use
- What safety precautions apply to estradiol transdermal gel and spray? A Guide to Safe Application and Risk Prevention
- What ongoing care is required while using transdermal estradiol? Ensure Safe & Effective Hormone Therapy















