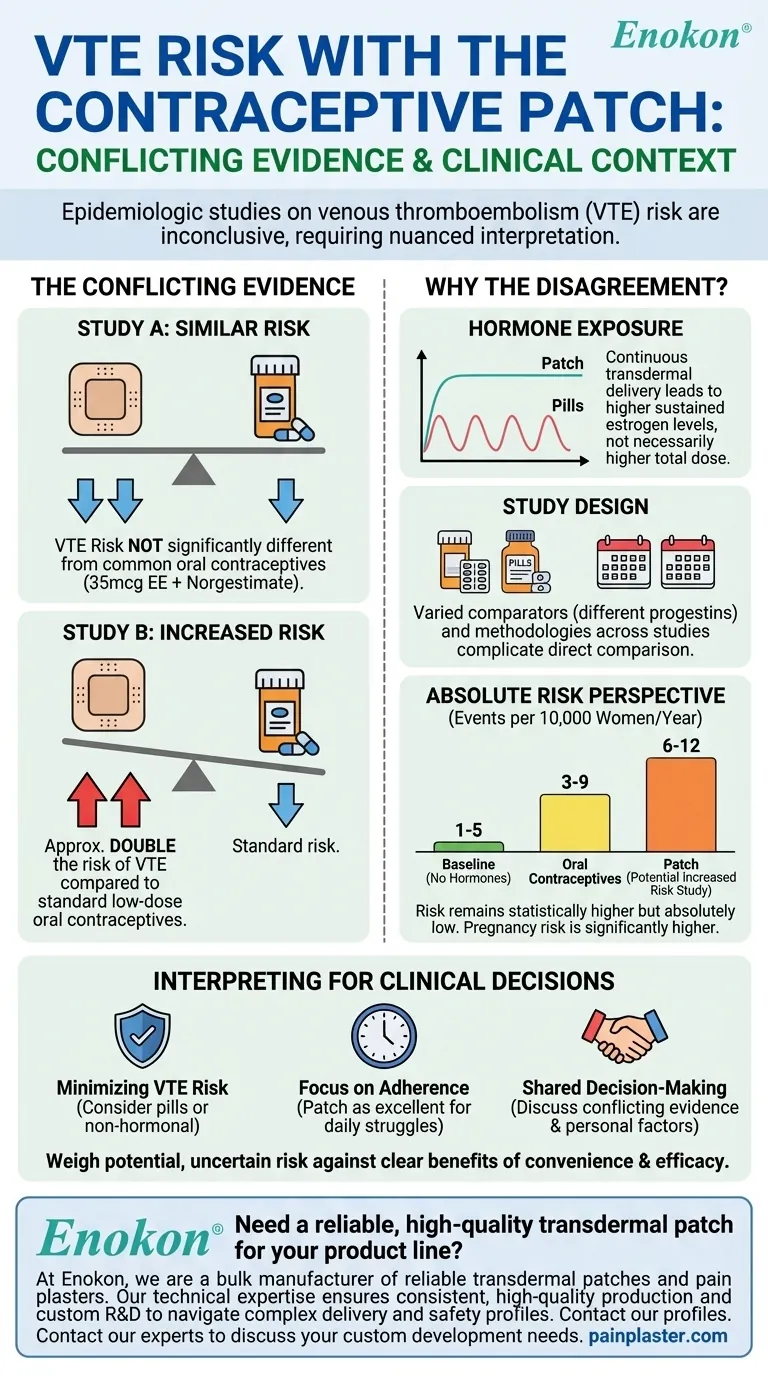
The short answer is that the evidence is conflicting. Two key epidemiologic studies examining the risk of venous thromboembolism (VTE) with the contraceptive patch produced different results. One study found a risk similar to that of common oral contraceptives, while the other suggested a potentially higher risk associated with patch use.
The core issue is not a definitive conclusion but rather a signal of uncertainty. The conflicting data highlights that while the patch is a highly effective contraceptive, its hormonal delivery system may alter VTE risk compared to traditional pills, warranting a careful risk-benefit analysis for each individual.
The Conflicting Epidemiologic Evidence
The discrepancy in findings stems from two large, post-marketing observational studies. Understanding both is key to interpreting the overall risk profile.
The "Similar Risk" Finding
One major study compared the VTE risk in new users of the patch to new users of oral contraceptives containing 35 mcg of ethinyl estradiol and norgestimate. This study concluded that the risk of non-fatal VTE was not significantly different between the two groups.
This finding suggested that, within the context of this particular study's design and population, the patch did not pose an elevated threat compared to a well-established oral contraceptive.
The "Increased Risk" Finding
In contrast, a second major study found a different result. This research suggested that users of the contraceptive patch had approximately double the risk of VTE compared to users of standard, low-dose oral contraceptives.
This study raised a significant safety signal, suggesting that some factor unique to the patch could be responsible for this increased risk.
Why Do the Studies Disagree?
The conflicting results are not necessarily a sign of flawed research but rather a reflection of the complexities of studying drug side effects in large populations. Several factors likely contribute to the disagreement.
Differences in Hormone Exposure
The primary hypothesis for a potential increased risk centers on estrogen exposure. While the patch contains a standard dose of estrogen, it is delivered transdermally and continuously.
This leads to a higher peak concentration and a more constant, steady-state level of estrogen in the bloodstream compared to the daily fluctuations seen with oral pills. This sustained exposure, not the dose itself, is thought to be the potential mechanism for a higher VTE risk.
Study Design and Comparators
The two studies used different methodologies and, crucially, different comparator groups. One study might compare the patch to a specific type of oral contraceptive, while another uses a broader range of pills.
The type of progestin in the comparator oral contraceptive is also a known factor that influences VTE risk, which can complicate direct comparisons. These subtle differences in study design can easily lead to varied outcomes.
Putting Absolute Risk into Perspective
It is critical to distinguish between relative risk (e.g., "double the risk") and absolute risk (the actual chance of an event occurring).
Baseline VTE Risk
For healthy, non-pregnant women of reproductive age who are not using hormonal contraception, the baseline risk of a VTE is very low—approximately 1 to 5 events per 10,000 women per year.
Risk with Oral Contraceptives
Using a combined oral contraceptive increases this risk to approximately 3 to 9 events per 10,000 women per year. The risk is still small but represents a clear increase over baseline.
Contextualizing the Patch Risk
The study suggesting a "doubled risk" would place the absolute risk for patch users at around 6 to 12 events per 10,000 women per year. While this is statistically higher, the absolute number of women who would experience a VTE remains very small. For context, the risk of VTE during pregnancy and the postpartum period is significantly higher.
Interpreting the Risk for Clinical Decisions
The conflicting evidence does not provide a simple yes-or-no answer. Instead, it demands a nuanced, individualized approach to contraceptive counseling.
- If the primary focus is minimizing all potential VTE risk: The conflicting data may lead you to favor oral contraceptives or non-hormonal methods, especially for patients with pre-existing risk factors like obesity, smoking, or a family history of clots.
- If the primary focus is contraceptive adherence and efficacy: The patch may be an excellent choice for a patient who struggles with daily pill-taking, as the increased risk of VTE from an unintended pregnancy is far greater than the risk from the contraceptive itself.
- If the goal is shared decision-making: The most appropriate action is to transparently discuss the conflicting evidence, the difference between relative and absolute risk, and the patient's personal risk factors and preferences.
Ultimately, the available data requires you to weigh a potential, but uncertain, increase in VTE risk against the patch's clear benefits of convenience and high efficacy.
Summary Table:
| Study Finding | Key Takeaway |
|---|---|
| Similar Risk | Found no significant difference in VTE risk compared to specific oral contraceptives. |
| Increased Risk | Suggested patch users had approximately double the VTE risk vs. standard oral contraceptives. |
| Absolute Risk | For healthy women, the absolute risk remains low (approx. 6-12 events per 10,000 women/year). |
Need a reliable, high-quality transdermal patch for your product line?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures consistent, high-quality production. We offer custom R&D and development services to create a patch that meets your exact specifications, helping you navigate complex delivery and safety profiles with confidence.
Contact our experts today to discuss your custom transdermal patch development needs.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Natural Herbal Wormwood Patch Pain Plaster
- Prostate Pain Kidney Health Care Patch for Men
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin













