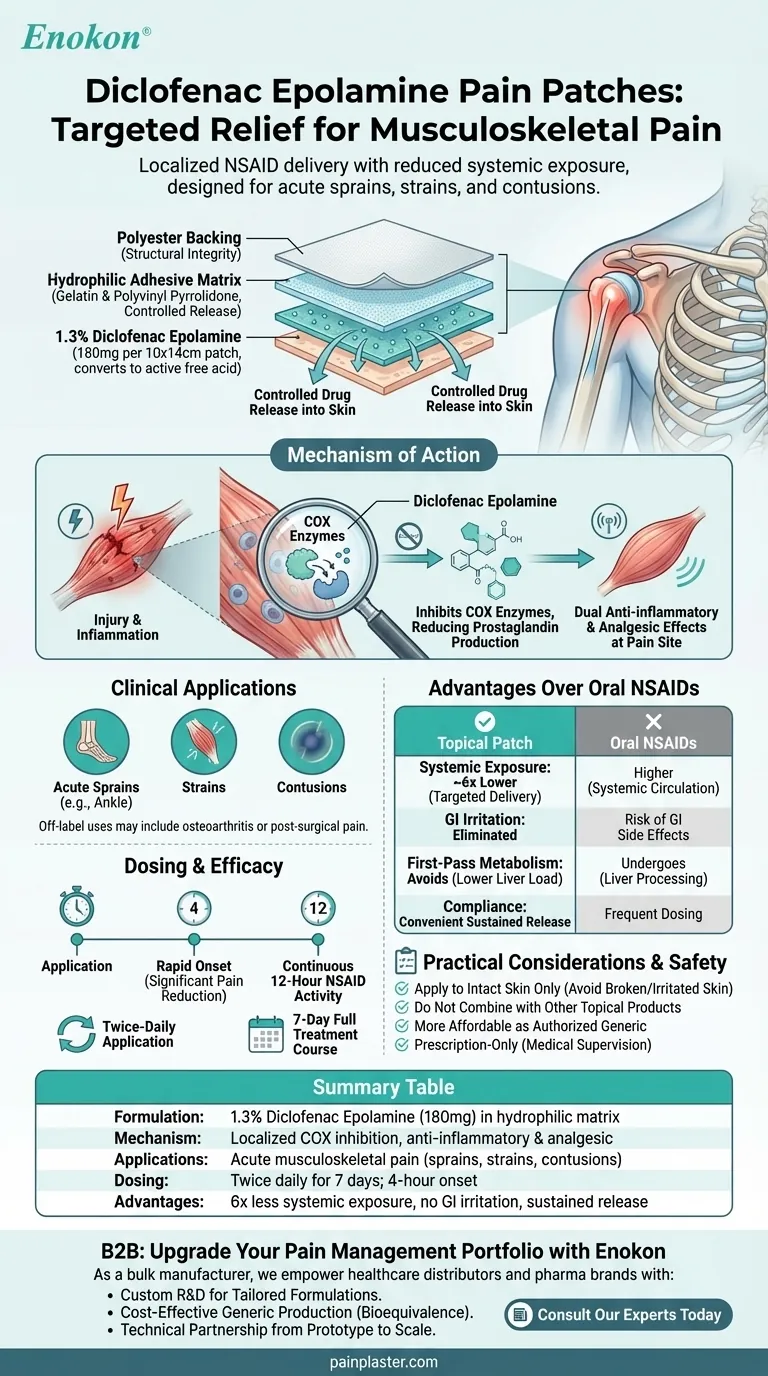
Diclofenac epolamine is a topical nonsteroidal anti-inflammatory drug (NSAID) formulated into pain patches for localized pain relief. These prescription patches deliver diclofenac directly to the affected area through intact skin, offering comparable efficacy to oral diclofenac while minimizing systemic exposure. The patches are specifically designed for acute musculoskeletal pain like sprains, strains, and contusions, providing both rapid (within 4 hours) and sustained (up to 7 days with twice-daily application) pain relief through a specialized adhesive matrix containing 1.3% diclofenac epolamine (equivalent to 1% free acid) on a polyester backing.

Key Points Explained:
-
Composition & Formulation
- Contains 1.3% diclofenac epolamine (180 mg per patch), a salt form converting to active diclofenac free acid
- Hydrophilic adhesive matrix with gelatin and polyvinyl pyrrolidone for controlled drug release
- Polyester backing material ensures structural integrity during wear
- 10 × 14 cm patch size optimizes surface area for drug delivery
-
Mechanism of Action
- NSAID that inhibits cyclooxygenase (COX) enzymes, reducing prostaglandin production
- Provides dual anti-inflammatory and analgesic effects at the pain site
- Localized action minimizes gastrointestinal side effects common with oral NSAIDs
-
Clinical Applications
- Primarily for acute musculoskeletal pain: sprains (e.g., ankle), strains, and contusions
- Off-label uses may include osteoarthritis or post-surgical pain management
- Applied directly over intact skin at the pain site, unlike systemic transdermal patches
-
Dosing & Efficacy
- Twice-daily application maintains therapeutic effect
- Achieves significant pain reduction within 4 hours of first application
- Provides 12 hours of continuous NSAID activity per application
- Full treatment course typically lasts 7 days
-
Advantages Over Oral NSAIDs
- Targeted delivery reduces systemic drug exposure by ~6x compared to oral dosing
- Avoids first-pass metabolism, lowering liver processing demands
- Eliminates GI irritation risks associated with oral administration
- Convenient sustained-release format improves patient compliance
-
Practical Considerations
- Requires intact skin application—avoid broken or irritated skin
- Should not be combined with other topical products at application site
- More affordable as an authorized generic versus brand-name equivalents
- Prescription-only status ensures proper medical supervision
Have you considered how this targeted delivery system exemplifies the shift toward precision pain management? These patches represent a thoughtful convergence of pharmaceutical chemistry and patient-centric design—offering effective relief while addressing traditional NSAID limitations. Their development reflects growing demand for therapies that balance potency with reduced systemic burden, quietly transforming how we approach common musculoskeletal injuries.
Summary Table:
| Key Aspect | Details |
|---|---|
| Formulation | 1.3% diclofenac epolamine (180 mg/patch) in hydrophilic adhesive matrix |
| Mechanism | COX inhibition for localized anti-inflammatory & analgesic effects |
| Applications | Acute musculoskeletal pain (sprains, strains, contusions) |
| Dosing | Twice daily for 7 days; effects within 4 hours |
| Advantages vs Oral NSAIDs | 6x less systemic exposure, no GI irritation, sustained release |
Upgrade your pain management portfolio with precision transdermal solutions
As a bulk manufacturer of clinically validated pain patches, Enokon empowers healthcare distributors and pharma brands with:
- Custom R&D for tailored formulations (e.g., varying dosages or adhesive systems)
- Cost-effective generic production meeting strict bioequivalence standards
- Technical partnership from prototype to commercial-scale manufacturing
Let’s develop your next-generation topical analgesic—consult our experts today to discuss formulation targets, regulatory pathways, and MOQ options.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks












