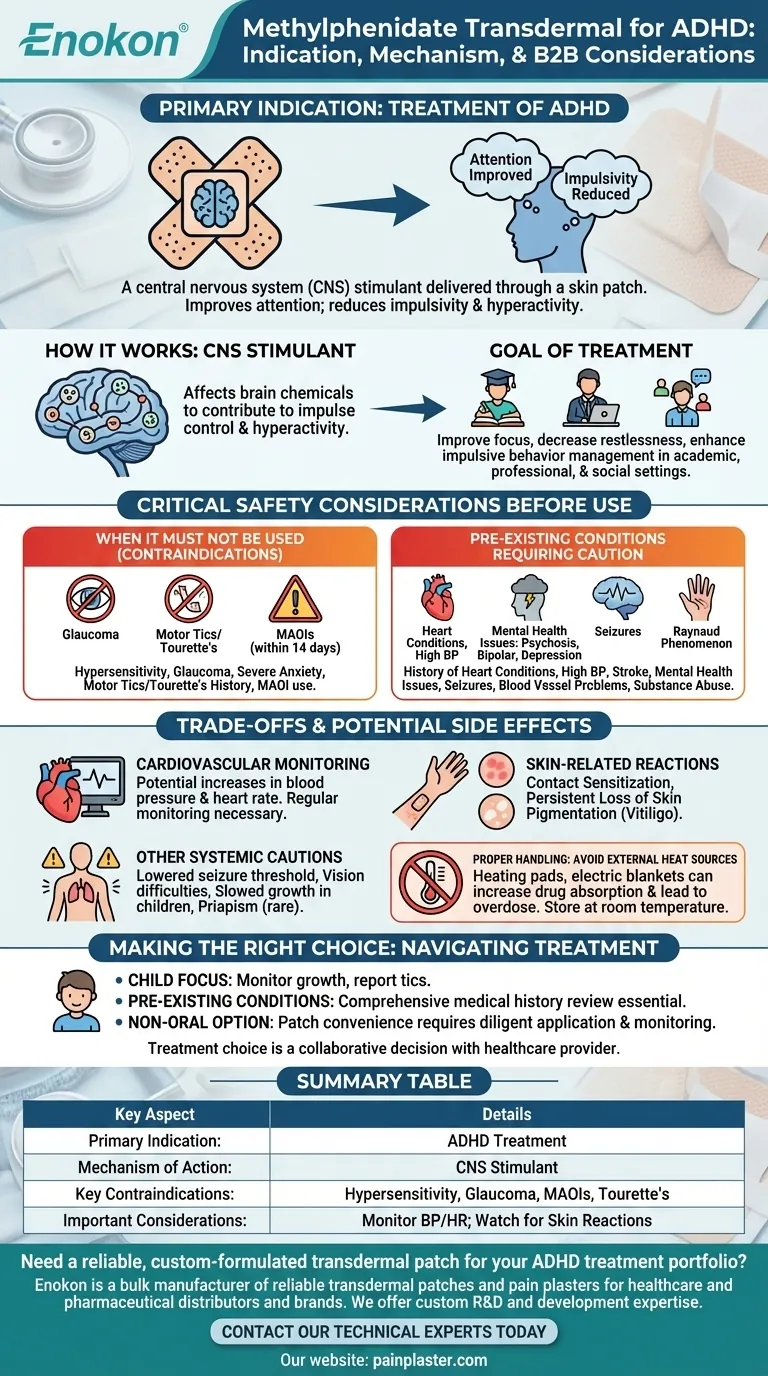
In short, methylphenidate transdermal is specifically indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). It is a central nervous system (CNS) stimulant delivered through a skin patch. The primary goal of this medication is to help improve attention while reducing the impulsivity and hyperactivity commonly associated with ADHD.
While methylphenidate transdermal is an established treatment for ADHD, its delivery method via a skin patch introduces unique considerations. Understanding the specific warnings, contraindications, and potential skin-related side effects is just as critical as understanding the medication's intended benefits.
How Methylphenidate Transdermal Works
A Central Nervous System Stimulant
Methylphenidate belongs to a class of medications known as CNS stimulants.
These medications work by affecting certain chemicals in the brain that contribute to impulse control and hyperactivity.
The Goal of Treatment
The intended outcome is to improve focus, decrease restlessness, and enhance a person's ability to manage impulsive behaviors.
This can lead to better performance in academic, professional, and social settings for individuals with ADHD.
Critical Safety Considerations Before Use
It is essential to have a thorough discussion with a healthcare provider before starting treatment, as certain conditions can make this medication unsafe or require close monitoring.
When It Must Not Be Used (Contraindications)
Methylphenidate transdermal is strictly contraindicated for individuals with certain conditions.
These include a known hypersensitivity to methylphenidate, glaucoma, or severe anxiety, tension, and agitation.
It must not be used by anyone with motor tics or a personal or family history of Tourette syndrome.
Furthermore, it cannot be used at the same time as monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping them.
Pre-existing Conditions Requiring Caution
Many other medical conditions require careful evaluation before using this medication.
Inform your doctor if there is a history of heart conditions, high blood pressure, or stroke.
A history of mental health issues like psychosis, bipolar disorder, or depression must be disclosed, as stimulants can sometimes worsen these conditions.
Other areas of concern include a history of seizures, blood vessel problems like Raynaud phenomenon, or substance abuse.
Understanding the Trade-offs and Potential Side Effects
Like any medication, the transdermal patch comes with a specific profile of potential side effects and necessary precautions.
Cardiovascular Monitoring
The medication can cause increases in blood pressure and heart rate. Regular monitoring by a healthcare professional is often necessary.
Skin-Related Reactions
Because the drug is delivered through the skin, specific reactions can occur at the application site.
This includes contact sensitization, an allergic skin reaction. More seriously, a persistent loss of skin pigmentation (vitiligo) has been reported at application sites.
Other Systemic Cautions
The medication has been associated with a lowered seizure threshold in patients with a history of seizures.
Other potential side effects include difficulties with vision, monitoring for slowed growth in children, and the rare but serious risk of prolonged and painful erections (priapism).
Proper Handling and Application
The application site should never be exposed to direct external heat sources, such as heating pads or electric blankets. This can increase the rate of drug absorption and lead to an overdose.
Patches must be stored at room temperature in their sealed pouches until ready for use.
Making the Right Choice for Your Goal
Navigating treatment for ADHD requires a clear understanding of the options and their specific implications.
- If your primary focus is on treating ADHD in a child: Be vigilant about monitoring growth and immediately report any development of tics to your doctor.
- If you have pre-existing health conditions: A comprehensive review of your full medical history, especially concerning heart, circulatory, or mental health, is absolutely essential before starting treatment.
- If your primary concern is finding a non-oral option: The patch offers this convenience but requires diligent adherence to application instructions and careful monitoring for any adverse skin reactions.
Ultimately, choosing the right treatment is a collaborative decision made with a healthcare provider, grounded in a full understanding of both the benefits and the risks.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Indication | Treatment of Attention Deficit Hyperactivity Disorder (ADHD) |
| Mechanism of Action | Central Nervous System (CNS) Stimulant |
| Key Contraindications | Hypersensitivity, glaucoma, use with MAOIs, Tourette's syndrome |
| Important Considerations | Monitor blood pressure/heart rate; watch for skin reactions at application site |
Need a reliable, custom-formulated transdermal patch for your ADHD treatment portfolio?
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. We offer custom R&D and development expertise to create the precise patch formulation you need.
Contact our technical experts today to discuss your project requirements and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- Is menthol topical safe during pregnancy and breastfeeding? Key Safety Insights
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
















