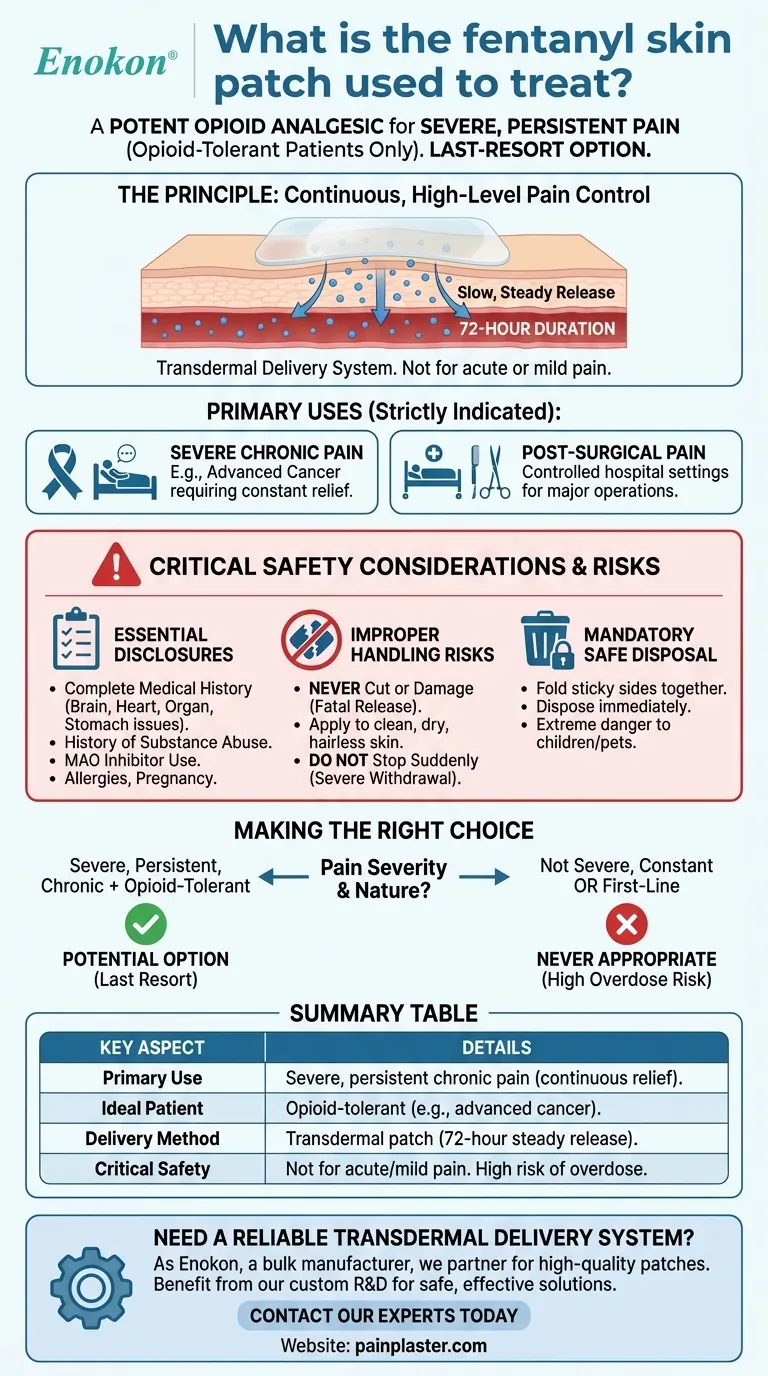
The fentanyl skin patch is a potent opioid analgesic used exclusively to treat severe, persistent pain that requires continuous, long-term, around-the-clock management. It is reserved for patients who are already "opioid-tolerant," meaning other pain medications are either no longer effective or cannot be tolerated.
The core takeaway is that fentanyl patches are not a starting point for pain relief. They are a powerful, last-resort option for severe, ongoing pain that cannot be managed by other means, and their use requires strict medical supervision due to significant risks.

The Principle: Continuous, High-Level Pain Control
Fentanyl patches are a transdermal delivery system, meaning the medication is absorbed through the skin. This design provides a slow, steady release of a powerful opioid over a 72-hour period.
When Is It Prescribed?
The patch is specifically indicated for two primary scenarios:
- Severe Chronic Pain: This includes pain from conditions like advanced cancer, where constant relief is necessary to maintain a quality of life.
- Post-Surgical Pain: In some controlled hospital settings, it may be used to manage severe, acute pain immediately following a major operation.
How It Delivers Medication
The patch is applied to the skin and must be pressed firmly for at least 30 seconds to ensure it adheres properly. It then slowly releases fentanyl into the bloodstream, providing a consistent level of pain medication for three full days.
Critical Safety Considerations and Risks
Due to its high potency, the fentanyl patch carries significant risks and is not appropriate for everyone. A thorough medical evaluation is non-negotiable before a prescription can be considered.
Essential Medical Disclosures
Before using a fentanyl patch, you must inform your healthcare provider about your complete medical history. This includes any history of:
- Brain tumors, head injuries, or seizures
- Heart, kidney, liver, or lung disease (including asthma)
- Stomach or intestine problems
- History of drug or alcohol abuse
- Use of an MAO inhibitor (like certain antidepressants) within the last 14 days
You must also disclose any allergies, especially to adhesives or other medications, and whether you are pregnant, planning to become pregnant, or breastfeeding.
Risks of Improper Handling
The patch contains a large amount of fentanyl that is meant to be released slowly. Any damage to the patch can cause a rapid, potentially fatal release of the medication.
- Never cut or damage a patch.
- Apply to clean, dry, and hairless skin on the chest, back, or upper arms.
- Do not stop using the patch suddenly. This can cause severe withdrawal. Discontinuation must be tapered under a doctor's supervision.
Safe Disposal is Mandatory
A used patch still contains enough medication to be extremely dangerous, especially to children or pets. After removal, a used patch should be folded so the sticky sides press together and then disposed of immediately according to your pharmacist's instructions.
Making the Right Choice for Your Goal
The decision to use a fentanyl patch is based entirely on the severity and nature of the pain, as well as the patient's medical history.
- If your primary focus is managing severe, persistent chronic pain: The patch is a potential option only if you are already opioid-tolerant and other long-term pain strategies are insufficient.
- If your primary focus is treating pain that is not severe or constant: The fentanyl patch is never the appropriate choice due to its high potency and the significant risk of overdose and dependence.
- If your primary focus is finding a first-line treatment for pain: This medication is considered a last resort and should only be discussed after all other viable pain management options have been exhausted.
Ultimately, the use of a fentanyl patch is a serious medical decision that must be guided by a qualified healthcare professional.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Use | Severe, persistent chronic pain requiring continuous, around-the-clock relief. |
| Ideal Patient | Opioid-tolerant individuals (e.g., advanced cancer pain). |
| Delivery Method | Transdermal patch providing a steady medication release over 72 hours. |
| Critical Safety Note | Not for acute, mild, or intermittent pain. High risk of overdose and dependence. |
Need a reliable transdermal delivery system for your pain management products?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors and brands. Our technical expertise ensures high-quality, consistent delivery systems for potent medications.
Benefit from our custom R&D and development services to create safe and effective solutions for your patients.
Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief













