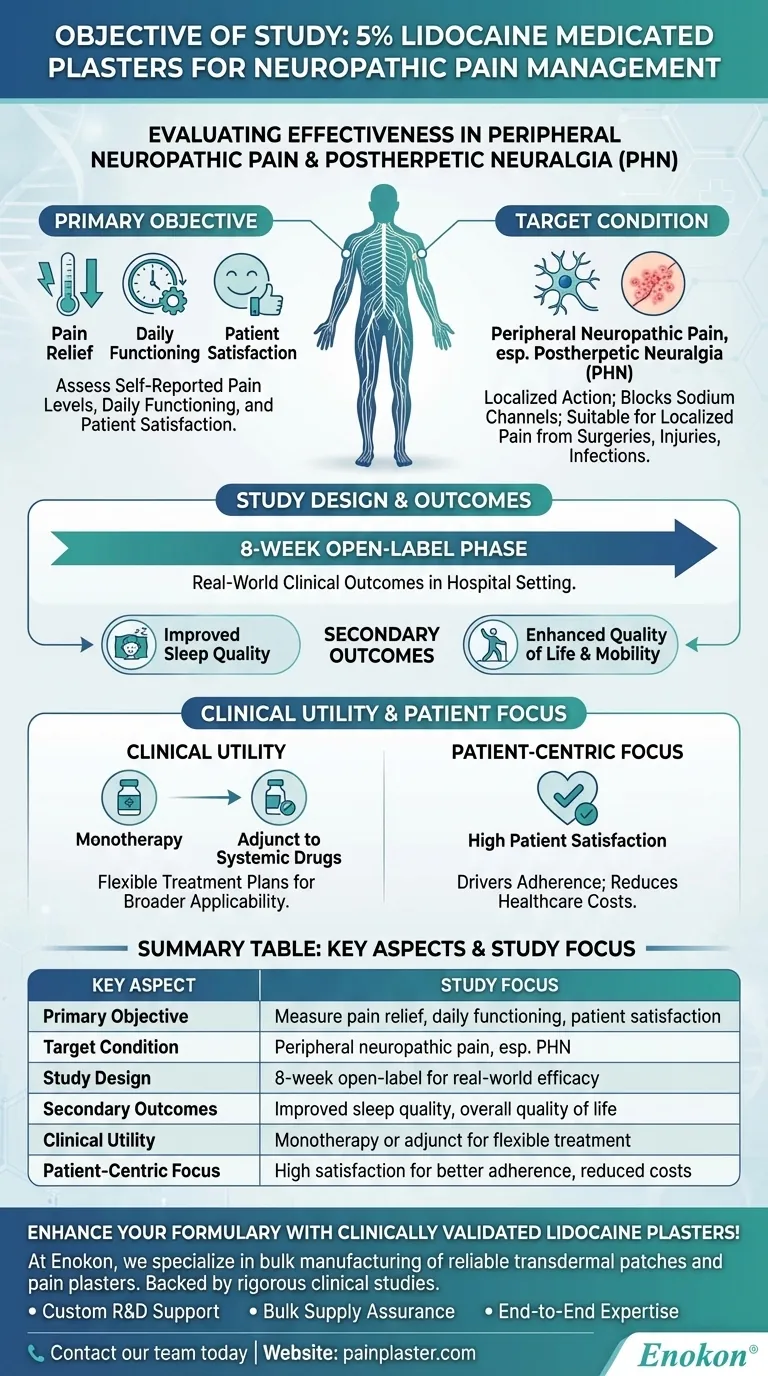
The study on 5% lidocaine medicated plasters aimed to evaluate their effectiveness in managing peripheral neuropathic pain, particularly postherpetic neuralgia (PHN), by assessing pain relief, functional improvement, sleep quality, and patient satisfaction. Conducted as an 8-week open-label arm of a larger controlled withdrawal study, it focused on real-world clinical outcomes in a hospital setting. The findings highlighted benefits beyond pain reduction, including enhanced quality of life and daily functioning, supporting the plaster's role as a monotherapy or adjunct treatment.

Key Points Explained:
-
Primary Objective:
- The study sought to measure the impact of 5% lidocaine plasters on self-reported pain levels, daily functioning, and patient satisfaction.
- Example: Patients with PHN often struggle with persistent pain; the plaster’s localized action was tested for its ability to alleviate this without systemic side effects.
-
Target Condition:
- Focused on peripheral neuropathic pain, especially postherpetic neuralgia (PHN), a complication of shingles causing chronic pain.
- The plaster’s mechanism—blocking sodium channels in damaged nerves—makes it suitable for localized neuropathic pain from surgeries, injuries, or infections like shingles.
-
Study Design:
- 8-week open-label phase: Patients received active treatment to observe real-world efficacy before transitioning to a blinded withdrawal phase.
- Why this matters: Open-label designs provide immediate clinical insights, while the subsequent controlled phase ensures rigor.
-
Secondary Outcomes:
- Beyond pain relief, the study evaluated sleep quality and quality of life—critical for chronic pain patients who often experience sleep disturbances and reduced mobility.
- Finding: Improved sleep and functionality underscored the plaster’s holistic benefits.
-
Clinical Utility:
- The plaster was tested as both monotherapy and adjunct to systemic drugs, offering flexibility in treatment plans.
- Practical implication: For purchasers, this versatility means broader applicability in formulary decisions.
-
Patient-Centric Focus:
- Emphasis on patient satisfaction reflected the growing need for treatments that align with patient preferences (e.g., non-invasive options).
- Takeaway: High satisfaction rates can drive adherence and reduce healthcare costs linked to ineffective therapies.
By addressing these dimensions, the study provided actionable data for clinicians and purchasers considering lidocaine plasters for neuropathic pain management.
Summary Table:
| Key Aspect | Study Focus |
|---|---|
| Primary Objective | Measure pain relief, daily functioning, and patient satisfaction. |
| Target Condition | Peripheral neuropathic pain, especially postherpetic neuralgia (PHN). |
| Study Design | 8-week open-label phase to assess real-world efficacy. |
| Secondary Outcomes | Improved sleep quality and overall quality of life. |
| Clinical Utility | Tested as monotherapy or adjunct to systemic drugs for flexible treatment. |
| Patient-Centric Focus | High patient satisfaction for better adherence and reduced healthcare costs. |
Enhance your pain management formulary with clinically validated lidocaine plasters!
At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors. Our 5% lidocaine medicated plasters are proven to improve pain relief, daily functioning, and patient satisfaction—backed by rigorous clinical studies.
Why choose Enokon?
- Custom R&D Support: Tailor formulations to meet specific patient or market needs.
- Bulk Supply Assurance: Dependable high-volume production for consistent inventory.
- End-to-End Expertise: From development to delivery, we optimize for efficacy and compliance.
📞 Contact our team today to discuss partnership opportunities or request samples!
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Detox Foot Patches for Detoxification
People Also Ask
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- What are common side effects of menthol patch? Key Risks & Safety Tips








