For pediatric patients, fentanyl patches are reserved exclusively for children aged two years and older who are already demonstrably opioid-tolerant. Due to the medication's high potency and the severe risk of life-threatening side effects, their use is governed by strict safety protocols and is never considered for children under two or for those new to opioid therapy.
The use of fentanyl patches in children is highly restricted because of the profound risk of fatal respiratory depression. Safe administration depends entirely on verifying prior opioid tolerance and preventing accidental exposure or heat-induced overdose.
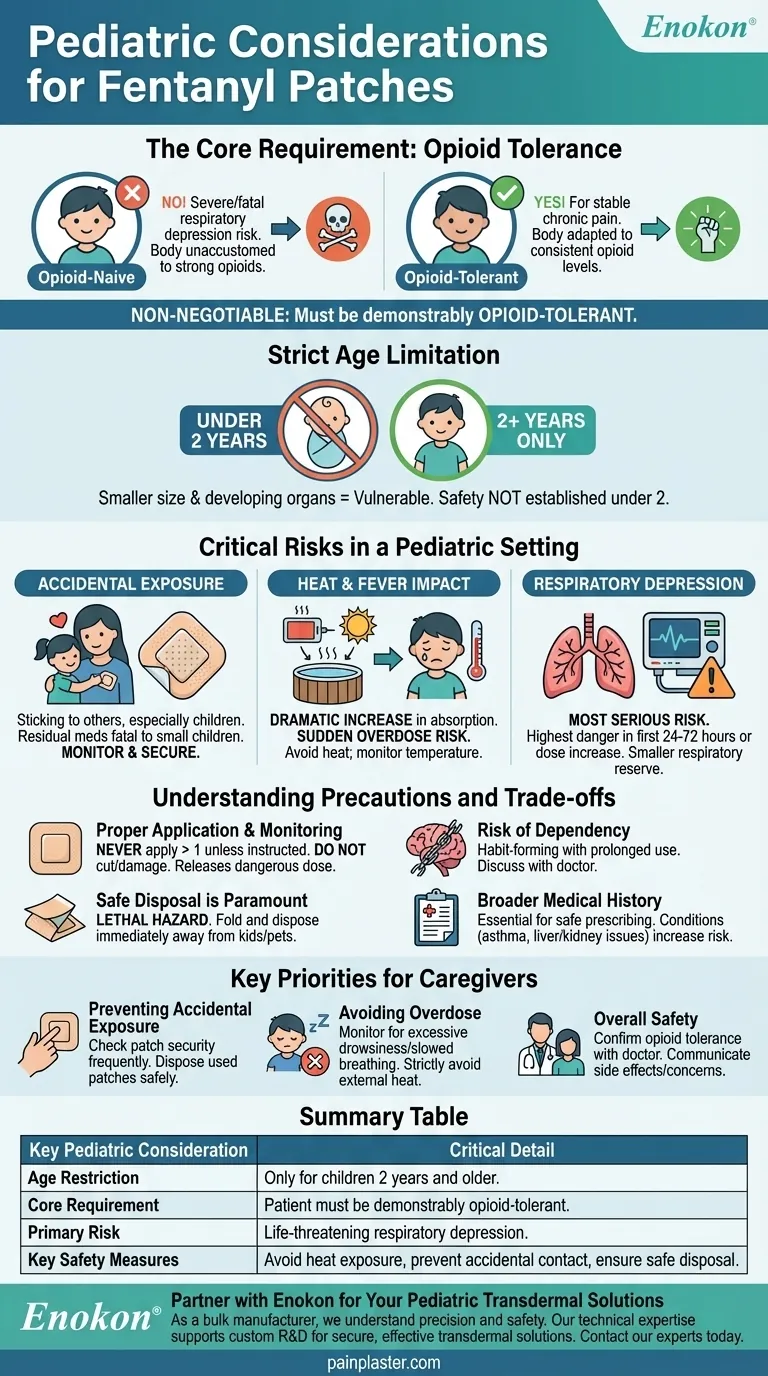
The Core Requirement: Opioid Tolerance
The central pillar of pediatric fentanyl use is the concept of opioid tolerance. A child's body must be accustomed to the effects of strong opioids before a fentanyl patch can even be considered.
Why Tolerance is Non-Negotiable
An "opioid-naive" patient—someone whose system is not used to these medications—can experience severe or fatal respiratory depression from a standard fentanyl dose.
Tolerance means the child's body has adapted to a consistent level of opioids, allowing them to handle the potency of fentanyl without being overwhelmed. This is why these patches are only used for managing severe, chronic pain in children already on a stable opioid regimen.
The Strict Age Limitation
Safety and effectiveness have not been established for children under the age of two. Their smaller body size and developing organ systems make them exceptionally vulnerable to the drug's potent effects.
Critical Risks in a Pediatric Setting
Beyond the need for tolerance, caregivers must be hyper-vigilant about the unique risks fentanyl patches pose to children and those around them.
The Danger of Accidental Exposure
A fentanyl patch can accidentally stick to another person, especially a child, during close contact like hugging. Even a used patch contains enough residual medication to be fatal to a small child who might find and touch or ingest it.
The Impact of Heat and Fever
Exposing the patch to external heat sources—like heating pads, hot tubs, or even direct sunlight—dramatically increases the rate of fentanyl absorption into the bloodstream.
This can trigger a sudden, life-threatening overdose. A child’s fever can produce the same effect, making it critical to monitor their temperature closely.
Life-Threatening Respiratory Depression
The most serious risk is severe breathing problems. This danger is highest during the first 24 to 72 hours of starting treatment or any time the dose is increased. A child’s smaller respiratory system has less reserve, making any suppression of breathing more dangerous.
Understanding the Precautions and Trade-offs
Using a fentanyl patch requires accepting significant risks in exchange for powerful pain relief. Managing these risks is a primary responsibility for any caregiver.
Proper Application and Monitoring
Never apply more than one patch unless specifically instructed by a physician. You must not cut or damage the patch in any way, as this can release a dangerously high dose of medication all at once.
The Risk of Dependency
Like with any strong opioid, fentanyl patches can be habit-forming, especially with prolonged use. This is a critical factor to discuss with the prescribing doctor.
Safe Disposal is Paramount
Used patches are a lethal hazard. They must be folded in half with the sticky sides together and immediately disposed of in a way that is inaccessible to children or pets.
Broader Medical History
A child's complete medical history is essential for safe prescribing. Conditions like breathing problems (asthma), head injuries, or impaired liver or kidney function can significantly alter how the body processes fentanyl, increasing risk.
Key Priorities for Caregivers
Navigating the use of fentanyl patches requires prioritizing safety above all else. Your focus determines your most critical actions.
- If your primary focus is preventing accidental exposure: Check frequently to ensure the patch is secure on the patient, and dispose of used patches immediately and safely.
- If your primary focus is avoiding overdose: Monitor the child for excessive drowsiness or slowed breathing, and strictly avoid all external heat sources near the patch.
- If your primary focus is overall safety: Ensure the prescribing doctor has confirmed the child is truly opioid-tolerant and communicate openly about any side effects or concerns.
Strict adherence to these safety protocols is the only way to manage severe pain in eligible children while mitigating the profound risks of fentanyl.
Summary Table:
| Key Pediatric Consideration | Critical Detail |
|---|---|
| Age Restriction | Only for children 2 years and older. |
| Core Requirement | Patient must be demonstrably opioid-tolerant. |
| Primary Risk | Life-threatening respiratory depression. |
| Key Safety Measures | Avoid heat exposure, prevent accidental contact, ensure safe disposal. |
Partner with Enokon for Your Pediatric Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon understands the critical need for precision and safety in pediatric applications. Our technical expertise supports healthcare and pharma distributors and brands with custom R&D and development to meet stringent safety standards.
Let us help you develop secure, effective transdermal solutions for your most vulnerable patients. Contact our experts today to discuss your specific needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
















