Before starting transdermal estradiol, you must have a comprehensive discussion with your healthcare provider about your complete medical history, any other medications or supplements you take, and relevant life circumstances. This includes discussing any history of heart problems, blood clots, or cancer, as well as any plans for surgery, pregnancy, or breastfeeding. This conversation is essential for ensuring the medication is safe and appropriate for you.
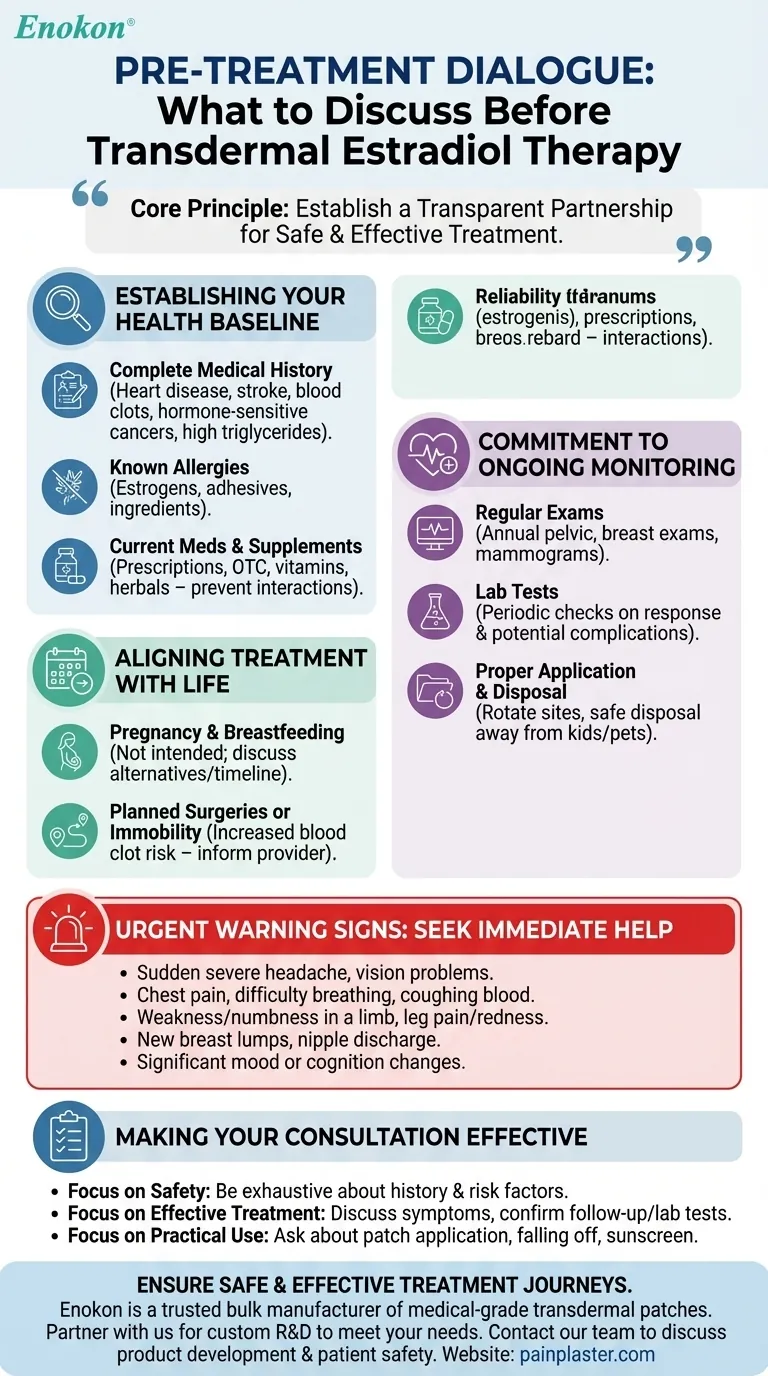
The core principle for safely using transdermal estradiol is establishing a transparent partnership with your provider. Effective treatment depends not just on the medication itself, but on a shared understanding of your unique health profile, lifestyle, and commitment to ongoing monitoring.

Establishing Your Health Baseline
The initial conversation is about creating a complete and accurate picture of your health. Your provider uses this information to assess risks and determine if this treatment is the right fit.
Your Complete Medical History
You must disclose your full health history, paying special attention to certain conditions.
Estrogen can impact cardiovascular health and cell growth, so it is critical to report any personal or family history of heart disease, stroke, blood clots, or hormone-sensitive cancers (like breast or uterine cancer). You should also mention conditions like high triglyceride levels.
Known Allergies and Sensitivities
An allergic reaction can be serious. Inform your doctor about any known allergies to estrogens, medical adhesives, or any other ingredients that might be in the patch.
All Current Medications and Supplements
Provide an exhaustive list of everything you take, including prescriptions, over-the-counter drugs, vitamins, and herbal products.
Many substances can interact with estradiol, potentially altering its effectiveness or increasing the risk of side effects. This transparency prevents dangerous drug interactions.
Aligning Treatment with Your Life Circumstances
Your daily life and future plans can significantly impact the safety and appropriateness of this therapy. These factors must be part of the discussion.
Pregnancy and Breastfeeding Plans
Estradiol is not intended for use during pregnancy or while breastfeeding. Be clear about your plans, as your provider will need to discuss alternative options or a timeline for stopping treatment.
Planned Surgeries or Immobility
If you have an upcoming surgery or anticipate a period of prolonged bedrest, you must inform your doctor. Immobility increases the risk of developing blood clots, a risk that can be compounded by estrogen therapy.
Understanding the Commitment to Monitoring
Using transdermal estradiol is not a one-time decision; it is a commitment to a monitored treatment plan. Understanding these long-term requirements is crucial for your safety.
The Necessity of Regular Physical Exams
You will need regular check-ups, typically at least once a year, to monitor your body's response to the medication. These appointments will include a pelvic exam, breast exam, and likely a mammogram based on standard screening guidelines.
The Role of Laboratory Tests
Your provider may order periodic lab tests. These tests provide objective data to check how your body is responding to the hormone and to screen for any potential complications before they become serious.
Proper Application and Disposal
Discuss the practical aspects of using the patch. This includes rotating application sites to prevent skin irritation and understanding the correct procedure for disposing of used patches to keep them away from children and pets.
Recognizing Urgent Warning Signs
A critical part of your discussion should be learning to identify symptoms that require immediate medical help. Knowing what to watch for empowers you to act quickly.
Symptoms Requiring Immediate Attention
Your provider will explain specific red flags. You must seek immediate medical care if you experience a sudden severe headache, vision problems, chest pain, difficulty breathing, coughing up blood, weakness or numbness in a limb, or pain and redness in your leg.
Other urgent signs include new breast lumps, nipple discharge, or significant changes in mood or cognition.
Making Your Consultation Effective
Your goal is to leave your appointment feeling confident and fully informed. Use these points to guide your conversation.
- If your primary focus is safety: Be exhaustive about your personal and family medical history, ensuring your provider has the complete picture to assess your risk factors.
- If your primary focus is effective treatment: Come prepared to discuss your symptoms in detail and confirm your willingness to attend all follow-up appointments and complete necessary lab tests.
- If your primary focus is practical day-to-day use: Ask specific questions about patch application, what to do if a patch falls off, and how lifestyle factors like sunscreen might affect absorption.
A thorough and honest conversation with your provider is the foundation of a safe and successful treatment plan.
Summary Table:
| Key Discussion Topic | Why It's Important |
|---|---|
| Full Medical History | To assess risks related to heart disease, blood clots, or hormone-sensitive cancers. |
| Current Medications & Supplements | To prevent dangerous drug interactions that alter effectiveness or increase side effects. |
| Pregnancy/Breastfeeding Plans | Estradiol is not safe during pregnancy or breastfeeding; alternatives must be discussed. |
| Planned Surgery or Immobility | To manage an increased risk of blood clots during periods of limited movement. |
| Commitment to Regular Monitoring | Ensures ongoing safety through annual physical exams, breast exams, and lab tests. |
| Recognizing Warning Signs | Empowers you to seek immediate help for symptoms like chest pain, severe headache, or leg redness. |
Ensure a Safe and Effective Treatment Journey
For healthcare distributors and brands, providing patients with reliable, high-quality transdermal patches is paramount. Enokon is a trusted bulk manufacturer of medical-grade transdermal patches and pain plasters.
Partner with us to provide your patients with the highest standard of care. Our technical expertise supports custom R&D and development to meet your specific needs.
Contact our team today to discuss how we can support your product development and ensure patient safety.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief












