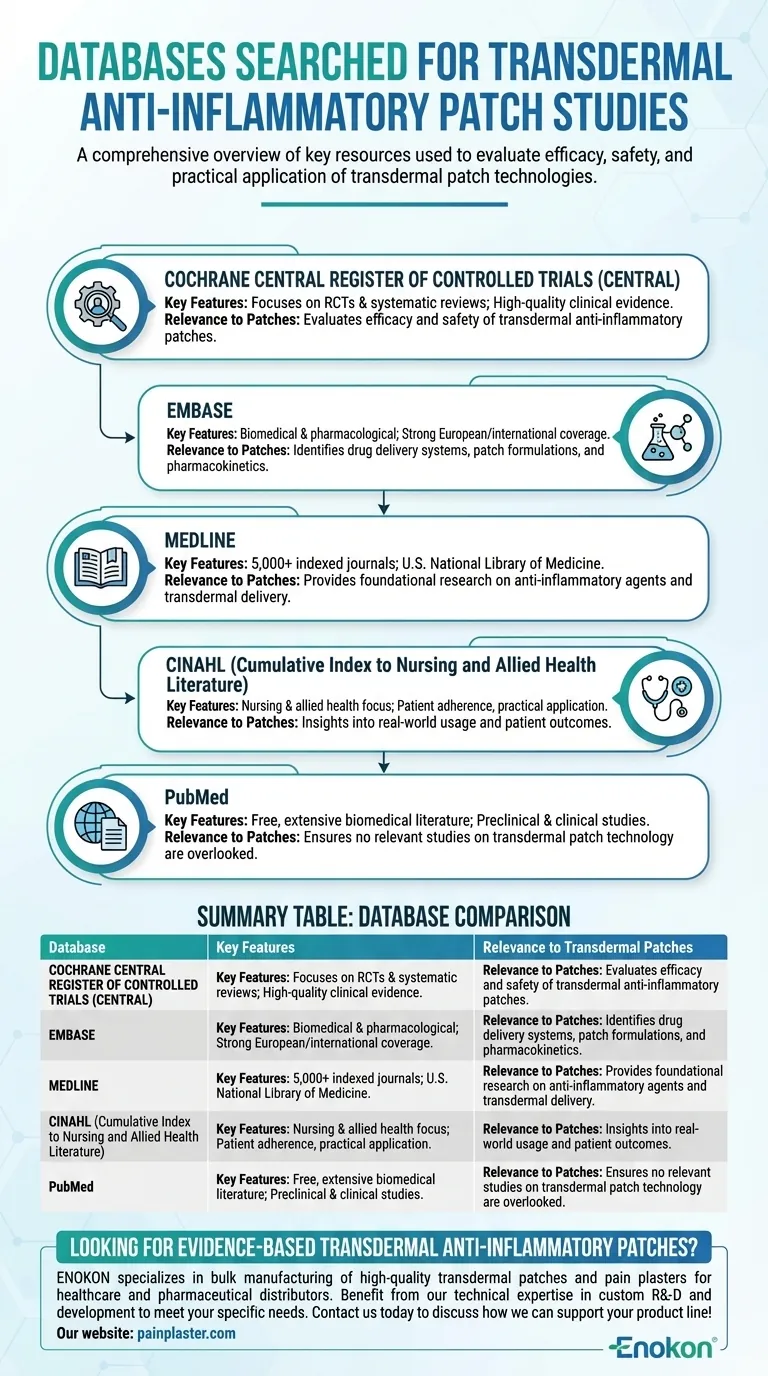
The databases searched for studies on transdermal anti-inflammatory patches included Cochrane Central Register of Controlled Trials, EMBASE, MEDLINE, CINAHL, and PubMed. These databases are widely recognized in medical and pharmaceutical research, providing comprehensive coverage of clinical trials, systematic reviews, and peer-reviewed literature. Their inclusion ensures a thorough examination of existing evidence on transdermal patch technologies and their anti-inflammatory applications.

Key Points Explained:
-
Cochrane Central Register of Controlled Trials (CENTRAL)
- A primary source for randomized controlled trials (RCTs) and systematic reviews.
- Critical for evaluating the efficacy and safety of transdermal anti-inflammatory patches, as it focuses on high-quality clinical evidence.
-
EMBASE
- A biomedical and pharmacological database with strong coverage of European and international literature.
- Useful for identifying studies on drug delivery systems, including transdermal patch formulations and their pharmacokinetics.
-
MEDLINE
- Maintained by the U.S. National Library of Medicine, indexing over 5,000 journals.
- Provides access to foundational research on anti-inflammatory agents and transdermal delivery mechanisms.
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature)
- Focuses on nursing and allied health research, including patient adherence and practical applications of patches.
- Offers insights into real-world usage and patient outcomes.
-
PubMed
- A free resource with extensive biomedical literature, including preclinical and clinical studies.
- Ensures no relevant studies on transdermal patch technology are overlooked.
These databases collectively provide a robust foundation for evidence-based conclusions about transdermal anti-inflammatory therapies. Their selection reflects a balance between clinical rigor (CENTRAL, MEDLINE), pharmacological depth (EMBASE), and practical healthcare perspectives (CINAHL). For purchasers, this breadth ensures informed decisions about patch efficacy, safety, and usability.
Summary Table:
| Database | Key Features | Relevance to Transdermal Patches |
|---|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL) | Focuses on RCTs and systematic reviews | Evaluates efficacy and safety of patches |
| EMBASE | Strong biomedical and pharmacological coverage | Identifies drug delivery systems and pharmacokinetics |
| MEDLINE | Indexes 5,000+ journals via U.S. National Library of Medicine | Provides foundational research on anti-inflammatory agents |
| CINAHL | Nursing and allied health focus | Insights into patient adherence and real-world usage |
| PubMed | Free, extensive biomedical literature | Ensures no relevant studies are overlooked |
Looking for evidence-based transdermal anti-inflammatory patches? Enokon specializes in bulk manufacturing of high-quality transdermal patches and pain plasters for healthcare and pharmaceutical distributors. Benefit from our technical expertise in custom R&D and development to meet your specific needs. Contact us today to discuss how we can support your product line!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism












