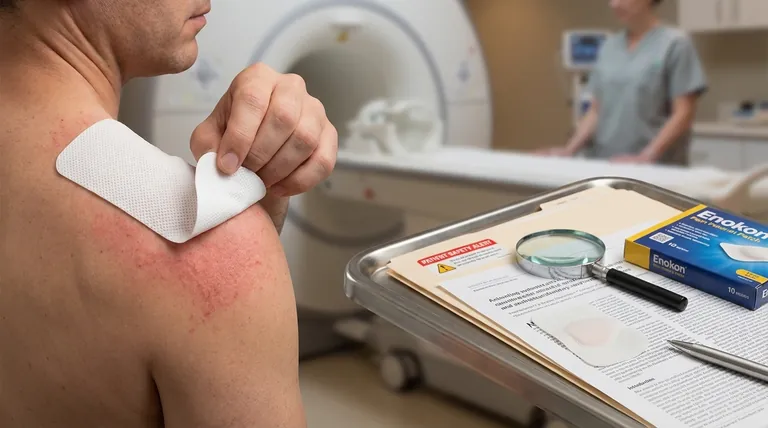
A Million-Dollar Question Before an MRI
A patient is being prepped for an urgent MRI. The technician runs through the standard checklist, but one question stands out: "Are you wearing any skin patches?"
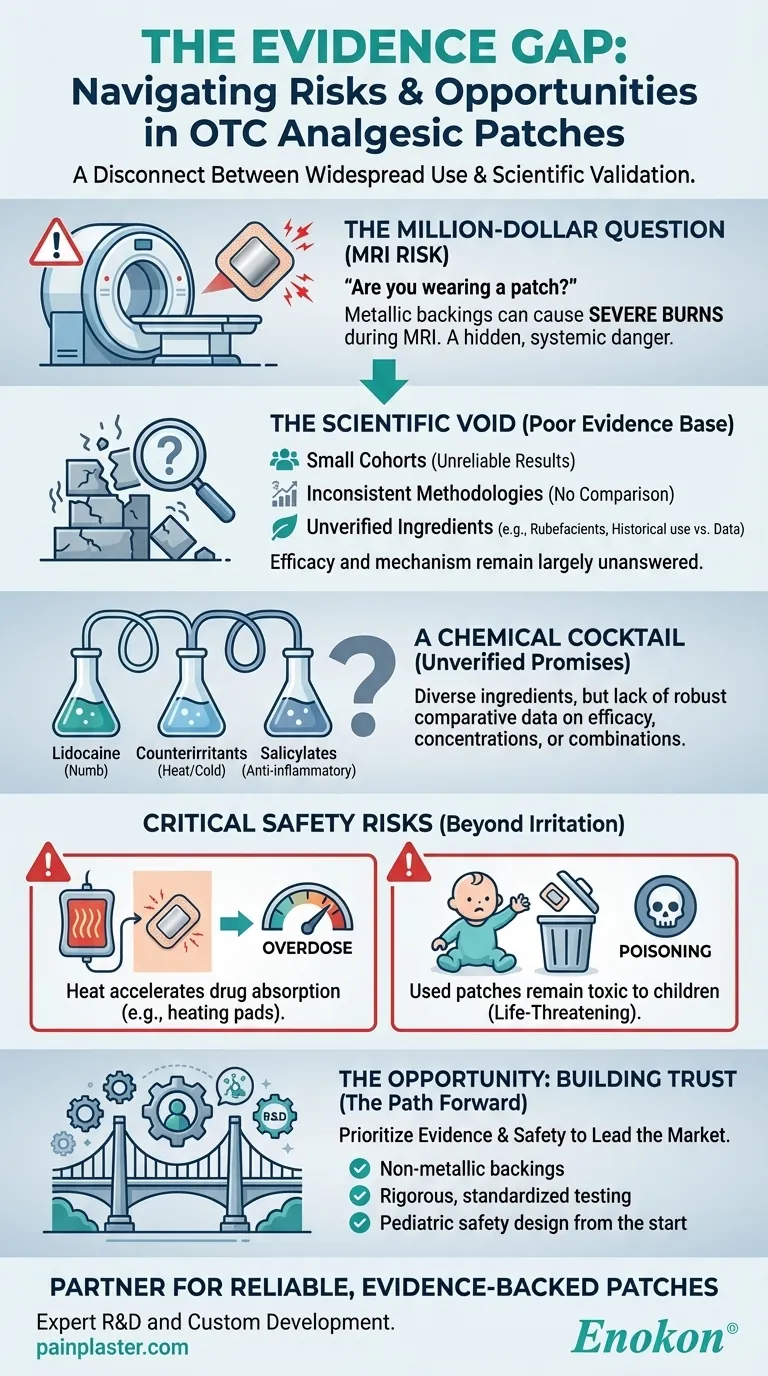
This isn't a trivial inquiry. A metallic-backed pain patch, a seemingly harmless OTC remedy, can cause severe burns during the scan. This single, specific danger reveals a much larger, systemic problem in the world of topical analgesics: a profound disconnect between widespread consumer use and rigorous scientific validation.
We trust these products implicitly, applying them for aches and pains without a second thought. Yet, the foundation of that trust is surprisingly fragile.
The Anatomy of a Scientific Void
The core issue isn't that OTC analgesic patches don't work; it's that we have remarkably poor-quality evidence to explain how or how well they work. The current body of research is plagued by systemic weaknesses.
- Small Patient Cohorts: Most studies are too small to yield statistically significant results, making their conclusions unreliable for broad populations.
- Inconsistent Methodologies: Without standardized trial designs, comparing one study to another is like comparing apples to oranges. This prevents the formation of a cohesive evidence base.
- The Rubefacient Riddle: Ingredients like methyl salicylate are ubiquitous, yet the clinical data supporting their efficacy in a patch format is minimal. Their inclusion is often based more on historical precedent than modern, controlled trials.
This gap creates a paradox. The market is saturated with products, yet the fundamental questions of efficacy and safety remain largely unanswered. For healthcare distributors and brands, this isn't just a scientific curiosity; it's a significant business risk.
A Chemical Cocktail with Unverified Promises
A typical analgesic patch contains a diverse portfolio of active compounds, each with a different mechanism of action.
- Local Anesthetics: Lidocaine to numb the area.
- Counterirritants: Capsaicin and menthol to create sensations of heat or cold that distract from pain.
- Salicylates: Methyl salicylate for anti-inflammatory effects.
This diversity looks impressive on a label. Psychologically, it suggests a multi-pronged attack on pain. But without robust comparative studies, it's just a chemical cocktail. We lack clear data on which ingredients are most effective, at what concentrations, and in which combinations. This ambiguity forces brands to rely on formulation "recipes" rather than evidence-based design.
From Skin Irritation to Systemic Danger
The lack of rigorous oversight extends directly to safety. While most side effects are minor, like skin irritation, the more severe risks are often overlooked until it's too late.
Hidden Hazards
Beyond the risk of MRI burns, proximity to external heat sources (like a heating pad) can dangerously accelerate drug absorption, leading to potential overdose. This is a critical design and labeling consideration that is often inadequately communicated.
The Pediatric Imperative
Perhaps the most sobering risk is to children. A used patch still contains a significant amount of its active ingredient. If found and ingested by a child, it can lead to acute, life-threatening poisoning. This places an immense ethical responsibility on manufacturers to design products and packaging that mitigate this danger.
Building Trust, One Patch at a Time
The evidence gap isn't a dead end; it's an engineering challenge and a market opportunity. For pharmaceutical brands and healthcare distributors, the path forward isn't to simply accept the status quo. It's to lead the market by prioritizing what has been neglected: evidence and safety.
This means moving beyond legacy formulations and investing in a higher standard. It means demanding patches with non-metallic backings, verifying ingredient efficacy through rigorous testing, and designing with pediatric safety in mind from the very beginning.
This is where a manufacturing partner with deep R&D expertise becomes indispensable. Instead of simply sourcing a generic product, you can collaborate to build a better one. Enokon specializes in exactly this—partnering with healthcare brands to develop and manufacture reliable, evidence-backed transdermal patches. Our technical expertise allows for custom development that addresses these critical safety and efficacy gaps, ensuring your product is built on a foundation of scientific trust, not market inertia.
The future of pain relief isn't just about discovering new molecules. It's about perfecting the tools we already have, closing the evidence gap, and earning the trust our customers place in us. To build a product that stands for safety and efficacy in a crowded market, Contact Our Experts.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Tube Cream for Pain Relief Analgesic Cream
Related Articles
- The Architecture of Relief: Deconstructing the Modern Pain Patch
- Beyond the Pill: The Engineering and Psychology of Transdermal Pain Relief in Dentistry
- The Precision Imperative: Why Topical NSAIDs Outsmart Oral Painkillers
- The Elegant Engineering of Targeted Pain Relief: Deconstructing the Ketoprofen TDS Patch
- Beyond the Molecule: The Behavioral Science of the Simple Pain Patch