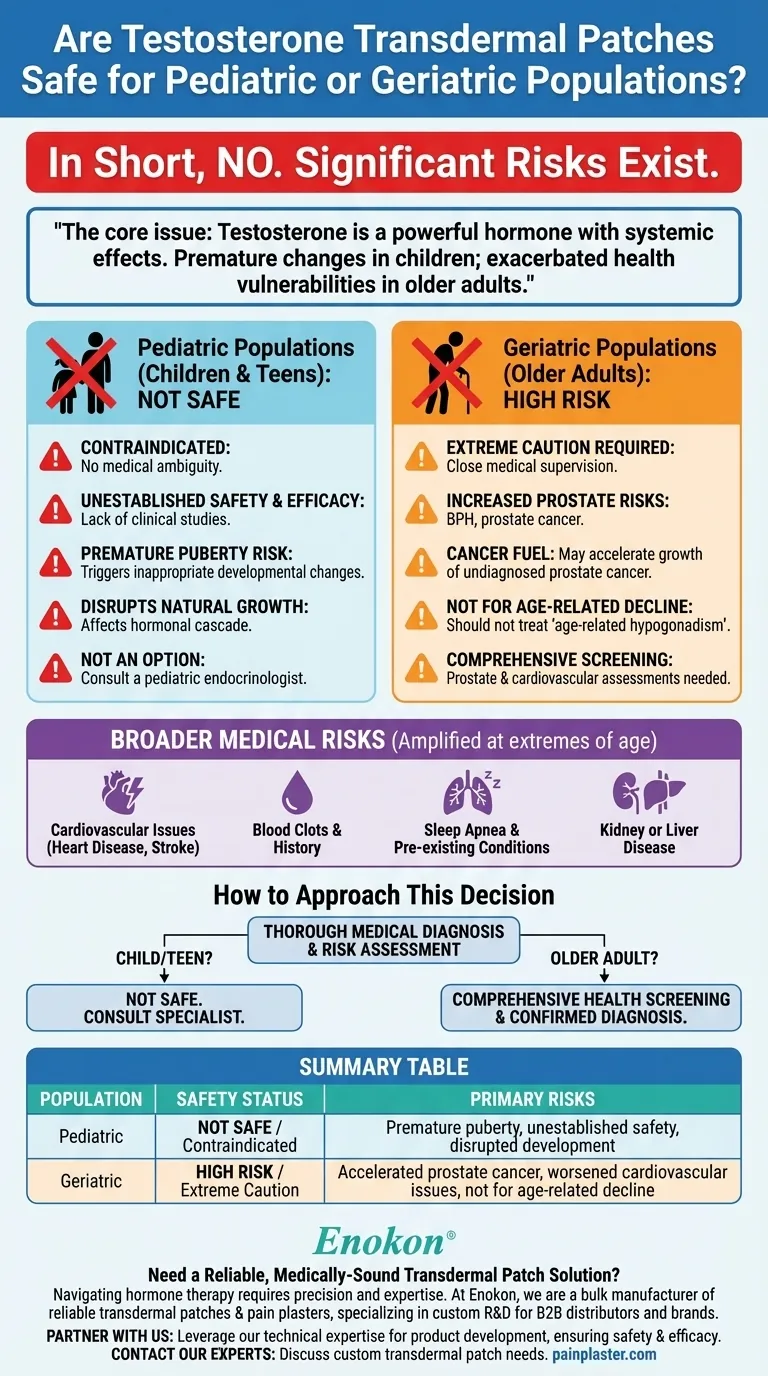
In short, no. Testosterone transdermal patches are not considered safe for pediatric use, and they pose significant risks requiring extreme caution in geriatric populations. The safety and effectiveness for children have not been established, while elderly patients face an increased risk of serious health complications, particularly related to the prostate.
The core issue is that testosterone is a powerful hormone with systemic effects. In children, it can cause premature and harmful developmental changes. In older adults, it can exacerbate age-related health vulnerabilities, such as prostate and cardiovascular conditions.

The Unestablished Safety in Children and Teenagers
The use of testosterone transdermal patches in pediatric populations is a clear and well-defined contraindication. There is no ambiguity on this point from a medical standpoint.
A Complete Lack of Safety Data
Appropriate clinical studies have never been performed to determine the relationship between age and the effects of testosterone patches in children or teenagers.
Because of this, both the safety and efficacy remain entirely unestablished for this age group.
The Danger of Premature Puberty
Introducing external testosterone to a developing body can trigger premature puberty. This can lead to a host of inappropriate physical changes and disrupt the natural, carefully timed hormonal cascade of adolescence.
Heightened Risks in Older Adults
While not absolutely contraindicated in all cases, the use of testosterone patches in geriatric patients is fraught with risks that demand careful consideration and close medical supervision.
Increased Risk of Prostate Problems
Elderly individuals are already at a higher baseline risk for developing prostate issues, including benign prostatic hyperplasia (BPH) and prostate cancer.
Testosterone can act as a fuel for certain prostate cancers. Applying it could potentially accelerate the growth of an undiagnosed cancer, making it a significant concern for this demographic.
Not a Treatment for Normal Aging
The references are clear that these patches should not be used to treat low testosterone levels that are a result of normal aging, often called age-related hypogonadism.
Their use is intended for specific medical conditions causing hypogonadism, not as a general anti-aging therapy.
Understanding the Broader Medical Risks
The concerns for pediatric and geriatric populations are amplified by the general risks associated with testosterone therapy, which can affect anyone but are more pronounced at the extremes of age.
The Impact of Pre-existing Conditions
The use of testosterone patches is complicated by numerous health issues that are more common in older adults.
Conditions like a history of blood clots, heart disease, stroke, kidney or liver disease, and sleep apnea can all be worsened by testosterone therapy.
Other Systemic Effects
Beyond specific conditions, testosterone can cause other significant side effects. These include decreased sperm production, which is a key consideration for reproductive health, and the potential for serious cardiovascular or mental health effects if the hormone is misused.
How to Approach This Decision
The choice to use testosterone therapy must be based on a clear medical diagnosis and a thorough evaluation of the risks versus the benefits for the specific individual.
- If you are considering this for a child or teenager: These patches are not a safe or appropriate option. You must consult with a pediatric endocrinologist to diagnose the underlying issue and explore medically established treatments.
- If you are an older adult considering this: Treatment should only proceed after a comprehensive health screening, including prostate and cardiovascular assessments, and a confirmed diagnosis of a specific medical condition, not just age-related decline.
Ultimately, testosterone therapy is a serious medical intervention that requires precise application and diligent monitoring by a qualified physician.
Summary Table:
| Population | Safety Status | Primary Risks |
|---|---|---|
| Pediatric (Children/Teens) | Not Safe / Contraindicated | Premature puberty, unestablished safety and efficacy, disruption of natural development |
| Geriatric (Older Adults) | High Risk / Extreme Caution Required | Accelerated prostate cancer growth, worsened cardiovascular issues, not for age-related decline |
Need a Reliable, Medically-Sound Transdermal Patch Solution?
Navigating the complexities of hormone therapy requires precision and expertise. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters, specializing in custom R&D for healthcare and pharmaceutical distributors and brands.
Partner with us to leverage our technical expertise for your product development. We ensure the highest standards of safety and efficacy for your target patient populations.
Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What class of drug is granisetron transdermal? A Guide to 5-HT3 Antagonist Patches
- How do transdermal patches provide versatility in drug delivery? Unlock Targeted, Sustained Release for Better Patient Outcomes
- What primary research challenge does HPLC address in transdermal drug delivery? Precise Trace Quantification
- When should you seek medical attention while using the birth control patch? Know the ACHES warning signs.
- What should be considered before using scopolamine transdermal patch? Key Safety & Application Factors
- What are symptoms of methylphenidate transdermal overdose? Recognizing a Critical Medical Emergency
- What should be done if the Ethinyl Estradiol; Norelgestromin patch falls off or is lost? Act Quickly to Maintain Protection
- What should be done if a dose of testosterone topical is missed? Managing Missed Doses Safely














