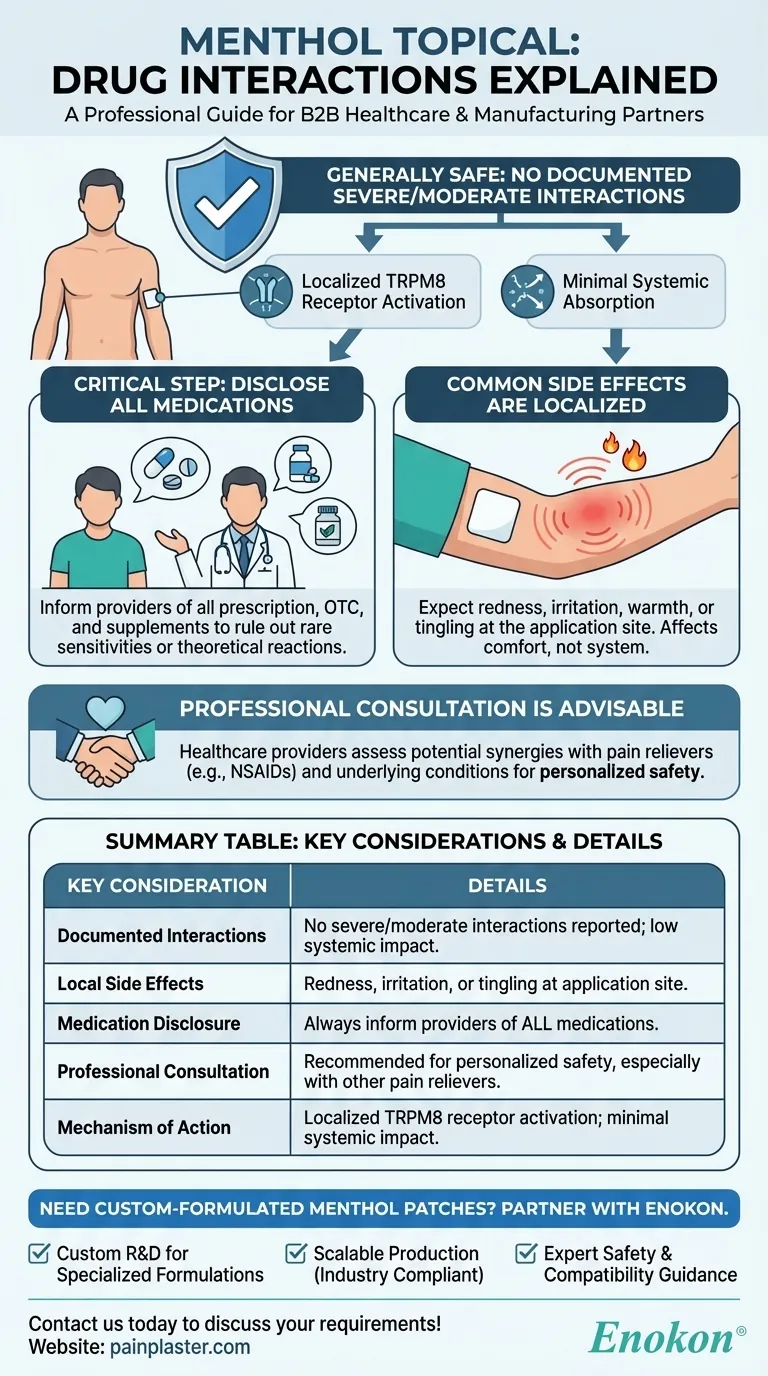
Menthol topical products, including menthol patches, are generally considered safe with no documented severe or moderate drug interactions. However, patients should always disclose all medications to healthcare providers due to potential individual sensitivities or rare reactions. Common side effects are typically localized to the application site, such as redness, irritation, or tingling. While current evidence suggests minimal systemic interactions, consulting a healthcare professional ensures personalized safety, especially when combining menthol topicals with other drugs.

Key Points Explained:
-
No Documented Severe/Moderate Interactions
- Multiple references confirm that menthol topical formulations (creams, gels, patches) have no clinically significant interactions classified as severe, serious, moderate, or mild.
- This suggests low systemic absorption and minimal interference with metabolic pathways used by other drugs.
-
Importance of Disclosing All Medications
- Despite the lack of documented interactions, patients should inform doctors about all medications (prescription, OTC, supplements) to rule out:
- Individual hypersensitivity reactions.
- Theoretical interactions with drugs affecting skin permeability or circulation (e.g., vasodilators).
- Despite the lack of documented interactions, patients should inform doctors about all medications (prescription, OTC, supplements) to rule out:
-
Localized Side Effects
- The primary risks involve application site reactions, such as:
- Erythema (redness), warmth, or irritation.
- Stinging/burning sensations or tingling.
- Rare hypersensitivity (allergic) responses.
- These are unlikely to interact systemically but may influence adherence or comfort.
- The primary risks involve application site reactions, such as:
-
Professional Consultation Advised
- While evidence suggests safety, healthcare providers can assess:
- Potential synergies with pain-relief medications (e.g., NSAIDs).
- Underlying conditions (e.g., sensitive skin, circulatory disorders) that may alter risk.
- While evidence suggests safety, healthcare providers can assess:
-
Mechanistic Considerations
- Menthol’s action as a TRPM8 receptor agonist (cooling sensation) is localized.
- Unlike oral menthol, topical application avoids first-pass metabolism, reducing interaction risks.
-
Gaps in Data
- Absence of documented interactions doesn’t guarantee universal safety.
- Rare cases or novel drug combinations may lack studies.
Always prioritize open communication with healthcare providers to tailor use to your specific regimen.
Summary Table:
| Key Consideration | Details |
|---|---|
| Documented Interactions | No severe/moderate interactions reported; low systemic absorption. |
| Local Side Effects | Redness, irritation, or tingling at application site. |
| Medication Disclosure | Always inform healthcare providers of all medications to assess risks. |
| Professional Consultation | Recommended for personalized safety, especially with other pain relievers. |
| Mechanism of Action | Localized TRPM8 receptor activation; minimal systemic impact. |
Need custom-formulated menthol patches or pain relief solutions?
At Enokon, we specialize in bulk manufacturing of high-quality transdermal patches and pain plasters for healthcare distributors and brands. Our technical expertise ensures reliable, safe, and effective products tailored to your needs.
✅ Benefits of partnering with us:
- Custom R&D for specialized formulations.
- Scalable production compliant with industry standards.
- Expert guidance on product safety and compatibility.
Contact us today to discuss your requirements!
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- What are common side effects of menthol patch? Key Risks & Safety Tips
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
















