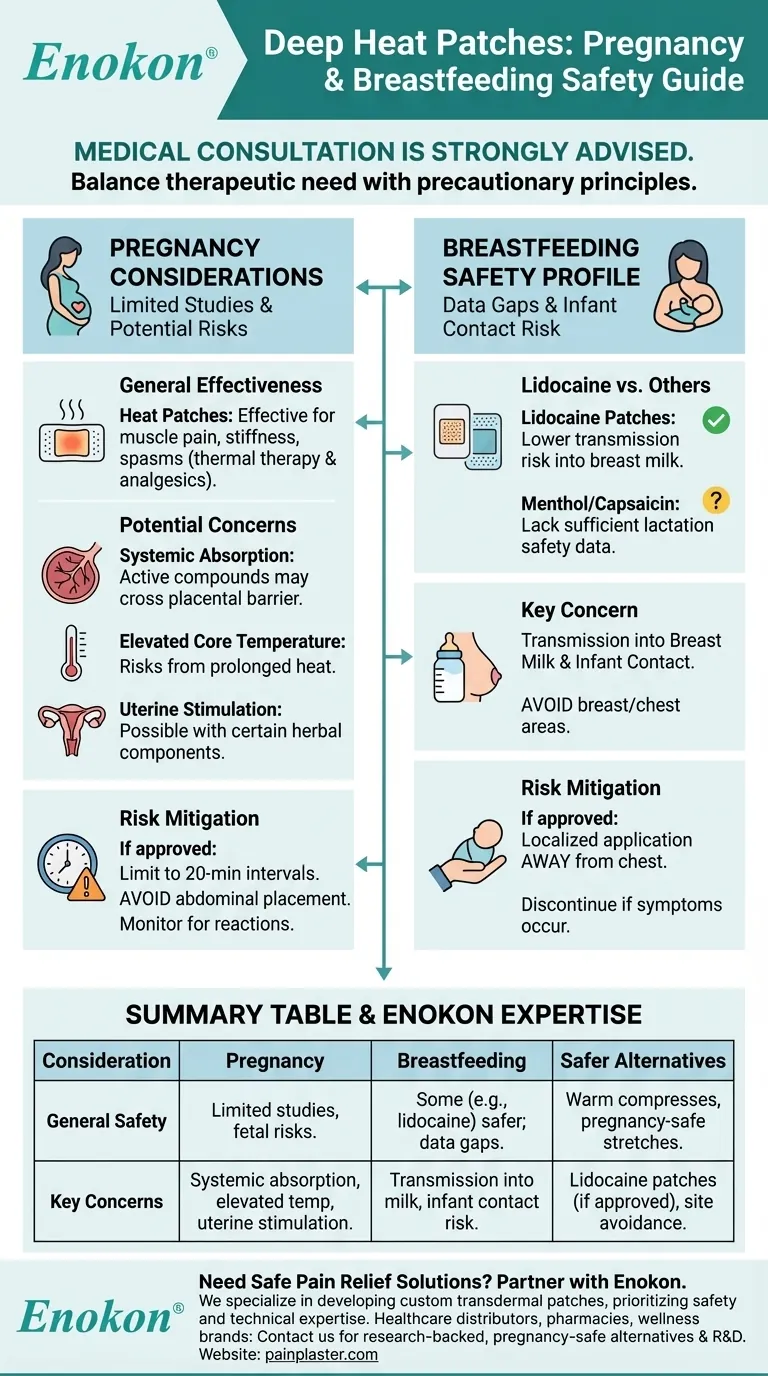
Deep Heat patches can provide relief for muscular pain and stiffness, but their use during pregnancy or breastfeeding requires careful consideration. While generally effective for pain management, the safety of specific ingredients for pregnant or nursing mothers isn't fully established. Medical consultation is strongly advised to evaluate potential risks versus benefits, as some components may affect fetal development or pass into breast milk. Lidocaine patches appear safer for breastfeeding based on available data, but this doesn't necessarily extend to all pain relief patches. The decision should balance therapeutic need with precautionary principles under professional guidance.

Key Points Explained:
-
General Effectiveness of Heat Patches
- Heat patches like Deep Heat are clinically proven for managing muscular pain, joint stiffness, and spasms through localized warmth and medication delivery
- Mechanism combines thermal therapy with topical analgesics (often methyl salicylate or menthol) to block pain signals and increase blood flow
-
Pregnancy-Specific Considerations
- No comprehensive studies confirm absolute safety of all patch ingredients during pregnancy
- Potential concerns include:
- Systemic absorption of active compounds crossing the placental barrier
- Elevated core temperature risks from prolonged heat application
- Possible uterine stimulation from certain herbal components
- First-trimester use carries higher theoretical risks due to critical fetal development
-
Breastfeeding Safety Profile
- Lidocaine patches demonstrate lower transmission risk into breast milk according to existing research
- Other common patch ingredients lack sufficient lactation safety data:
- Menthol transfer kinetics unstudied
- Capsaicin absorption potential unknown
- Application site matters (avoid breast/chest areas to prevent infant contact)
-
Critical Need for Medical Consultation
- OB/GYN or midwife can:
- Review exact product formulation
- Assess individual risk factors (e.g., high-risk pregnancy)
- Suggest safer alternatives like warm compresses or pregnancy-safe stretches
- Required even for "natural" patches due to potential herb-drug interactions
- OB/GYN or midwife can:
-
Risk Mitigation Strategies
- If approved by healthcare provider:
- Limit application to 20-minute intervals
- Avoid abdominal placement
- Monitor for adverse reactions (rash, dizziness, contractions)
- Discontinue immediately if unusual symptoms occur
- If approved by healthcare provider:
This nuanced approach ensures maternal comfort while prioritizing fetal/infant wellbeing through evidence-based decision making. Always verify current product formulations, as manufacturers may change ingredients.
Summary Table:
| Consideration | Pregnancy | Breastfeeding |
|---|---|---|
| General Safety | Limited studies; potential risks for fetal development | Some ingredients (e.g., lidocaine) may be safer; others lack sufficient data |
| Key Concerns | Systemic absorption, elevated core temperature, uterine stimulation | Transmission into breast milk, infant contact risk |
| Medical Advice Required? | Yes (consult OB/GYN or midwife) | Yes (consult healthcare provider) |
| Safer Alternatives | Warm compresses, pregnancy-safe stretches | Lidocaine patches (if approved), localized application away from chest |
| Risk Mitigation | Limit application time, avoid abdominal placement, monitor for reactions | Avoid breast/chest areas, discontinue if adverse symptoms occur |
Need safe pain relief solutions during pregnancy or breastfeeding? At Enokon, we specialize in developing custom transdermal patches tailored to your needs while prioritizing safety. Our technical expertise ensures formulations that align with medical guidelines.
Whether you're a healthcare distributor, pharmacy chain, or wellness brand, partner with us for reliable, research-backed pain relief products.
Contact our experts today to discuss pregnancy-safe alternatives or custom R&D solutions for your product line.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief


















