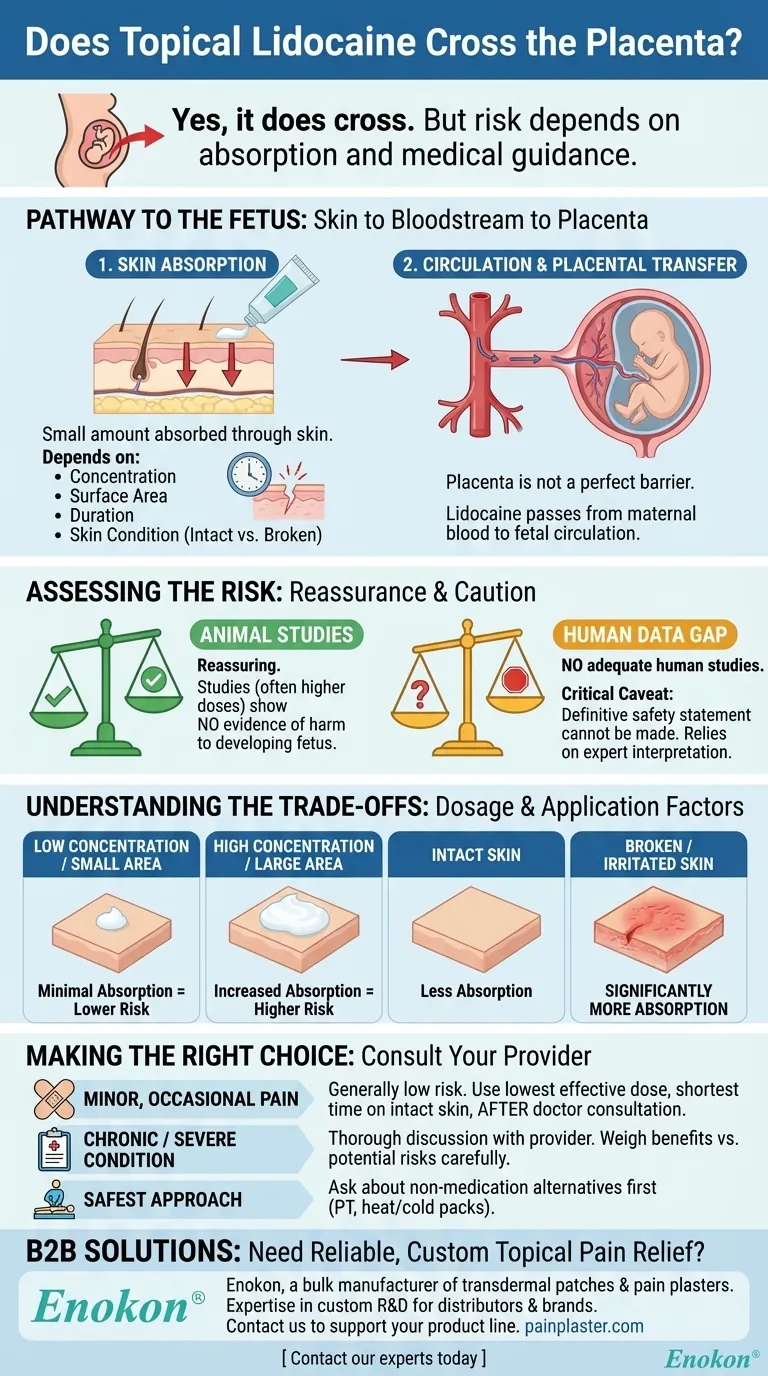
Yes, lidocaine applied topically can and does cross the placenta. Once a small amount of the medication is absorbed through the skin into the mother's bloodstream, it can be transferred to the fetus. However, it is crucial to understand that the presence of the drug does not automatically mean it causes harm.
The core issue is not simply that lidocaine crosses the placenta, but rather how much is absorbed and what the actual risk is. Based on current data, the risk is considered low when used correctly, but professional medical guidance is essential due to the lack of definitive human studies.

How Topical Lidocaine Reaches the Fetus
To understand the risk, you first need to understand the pathway from your skin to your baby. The process involves two key steps: systemic absorption and placental transfer.
From Skin to Bloodstream
Your skin acts as a barrier, but it is not impenetrable. When you apply a topical medication like lidocaine, a small portion of the active ingredient is absorbed through the skin and enters your circulatory system.
The amount absorbed depends heavily on several factors: the concentration of the lidocaine, the surface area it's applied to, the duration of application, and whether the skin is intact or broken.
The Placental Barrier
Once lidocaine is in your bloodstream, it circulates throughout your body. The placenta, while a protective barrier, is not a perfect filter. Many substances, including medications like lidocaine, can pass from the mother's blood to the fetal circulation.
Assessing the Actual Risk
The fact that lidocaine can reach the fetus is established. The more important question is whether that exposure is harmful.
What Animal Studies Suggest
The available evidence from animal reproduction studies is reassuring. These studies, which often involve higher doses than humans would typically use, have not shown evidence of harm to the developing fetus.
The Gap in Human Data
This is the critical caveat: there are no adequate and well-controlled studies on the use of topical lidocaine in pregnant women. For ethical reasons, this kind of research is rarely conducted.
This lack of direct human data means that a definitive statement on absolute safety cannot be made. Medical guidance relies on interpreting animal data and understanding the drug's properties.
Understanding the Trade-offs
Every medical decision during pregnancy is a balance of benefit and potential risk. Using topical lidocaine is no different.
The Impact of Dosage and Application
The risk of significant fetal exposure is directly tied to the amount of lidocaine that enters your system. Using a small amount of a low-concentration cream on a small patch of healthy, intact skin results in minimal absorption.
Conversely, applying a high-concentration product over a large area, especially on broken or irritated skin, will significantly increase systemic absorption and potential fetal exposure.
The Importance of Medical Context
Your healthcare provider is the only one who can properly weigh the variables. They will consider the reason you need pain relief, the severity of your symptoms, and your overall health profile.
Sometimes, the benefit of managing significant pain—which itself can cause stress and raise blood pressure—outweighs the very low theoretical risk of using topical lidocaine as directed.
Making the Right Choice for Your Pregnancy
Always consult your doctor or pharmacist before using any medication during pregnancy, including over-the-counter topical lidocaine.
- If your primary focus is treating occasional, minor pain: The risk is generally considered very low, but you must use the lowest effective dose for the shortest possible time on intact skin after consulting your doctor.
- If your goal is to manage a chronic or severe condition: This requires a thorough discussion with your healthcare provider to carefully evaluate the benefits versus the potential risks for your specific situation.
- If you are looking for the safest possible approach: Ask your doctor about non-medication alternatives for pain management first, such as physical therapy, heat, or cold packs.
Ultimately, making an informed decision with your healthcare provider is the safest path forward for both you and your baby.
Summary Table:
| Factor | Impact on Fetal Exposure |
|---|---|
| Concentration | Higher concentration increases absorption and potential exposure. |
| Application Area | Larger surface area leads to greater systemic absorption. |
| Skin Condition | Broken or irritated skin allows for significantly more absorption. |
| Duration of Use | Longer application times increase the total amount absorbed. |
Need reliable, custom-formulated topical pain relief solutions?
As Enokon, a bulk manufacturer of transdermal patches and pain plasters, we provide healthcare and pharmaceutical distributors and brands with technically advanced, consistent products. Our expertise in custom R&D ensures your formulations meet precise safety and efficacy standards.
Let's discuss your development needs. Contact our experts today to explore how we can support your product line.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How do lidocaine pain patches work and what are they used for? Targeted Pain Relief Without Systemic Side Effects
- How should nonprescription lidocaine transdermal be used? Safe & Effective Pain Relief Guide
- How is lidocaine transdermal patch used for postherpetic neuralgia pain? Get Targeted Relief Safely
- How effective are lidocaine patches for shingles pain? Targeted Relief with Minimal Side Effects
- What are the possible side effects of lidocaine plasters? Risks & Safety Tips
- How should missed doses of topical lidocaine be handled? A Safety Guide to Prevent Toxicity
- Are there any drug interactions with lidocaine 5 percent topical patch? Key Safety Insights
- What are the uses of lidocaine 5 percent topical patch? Targeted Pain Relief Without Systemic Risks

















