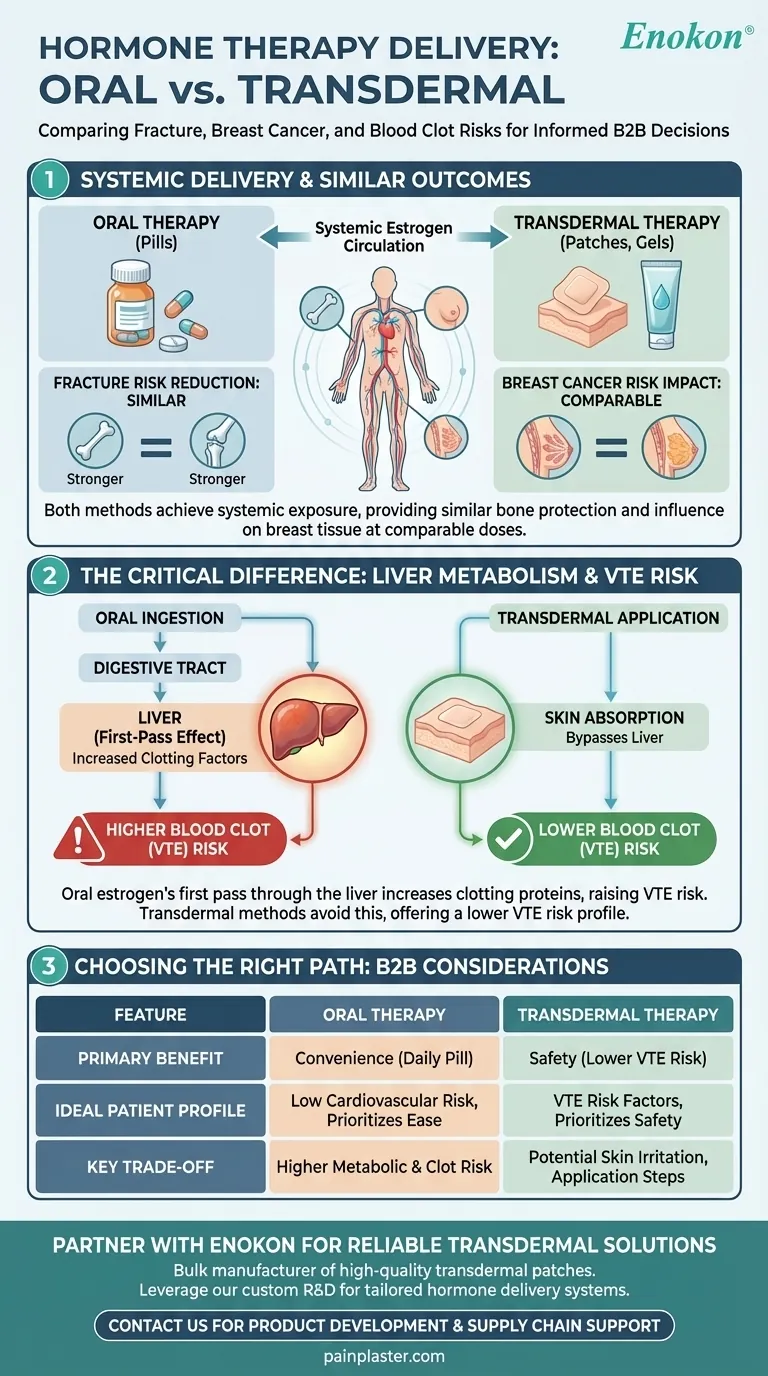
When considering menopausal hormone therapy, the method of delivery—oral pills versus transdermal patches or gels—is a critical factor for certain health risks. However, for reducing fracture risk and its impact on breast cancer risk, current evidence shows no significant difference between the two preparations. Both routes deliver estrogen systemically, providing similar bone protection and carrying a comparable influence on breast tissue.
The crucial takeaway is that while the choice between oral and transdermal hormone therapy is vital for managing risks like blood clots, it is not a deciding factor for the therapy's effects on bone density or breast cancer risk.

The Principle: Systemic Hormone Delivery
To understand why these specific risks are comparable, it's essential to recognize that both oral and transdermal methods are designed to achieve systemic exposure, meaning the hormone circulates throughout your entire body.
How Both Methods Protect Bones
Once estrogen enters the bloodstream, it travels to bone tissue. There, it helps slow the rate of bone breakdown, a process that accelerates after menopause. Because both oral and transdermal routes effectively raise systemic estrogen levels, they offer a similar degree of protection against osteoporotic fractures.
The Systemic Impact on Breast Tissue
Similarly, the risk of breast cancer associated with hormone therapy is linked to the stimulation of breast tissue by circulating estrogen. Since both delivery methods lead to this systemic exposure, their impact on breast cancer risk is considered equivalent when administered at comparable doses.
Where the Delivery Method Does Matter
The fundamental difference between oral and transdermal therapy lies in how the estrogen first enters your system. This initial path has significant implications for other health risks, particularly blood clots.
The "First-Pass Effect" of Oral Estrogen
When you take an estrogen pill, it is absorbed from your digestive tract and passes directly through the liver before entering general circulation. This "first-pass metabolism" causes the liver to produce higher levels of certain proteins, including those involved in blood clotting.
How Transdermal Therapy Bypasses the Liver
Transdermal methods, such as patches, gels, or sprays, deliver estrogen directly through the skin into the bloodstream. This route bypasses the initial pass through the liver, avoiding the significant increase in clotting factors seen with oral preparations.
The Critical Implication: Blood Clot Risk
This difference in liver metabolism is why transdermal hormone therapy is associated with a lower risk of venous thromboembolism (VTE), or blood clots in the veins, compared to oral therapy. This is the most significant safety distinction between the two delivery methods.
Understanding the Trade-offs
Choosing a delivery method involves balancing safety profiles with practical considerations. Neither route is universally superior; the right choice depends entirely on your individual health profile.
Oral Therapy: Convenience vs. Metabolic Risk
Oral pills are often seen as simple and convenient to take. However, due to the first-pass effect, they carry a higher metabolic burden, most notably the increased risk of blood clots. This makes them less suitable for individuals with pre-existing risk factors for VTE.
Transdermal Therapy: Safety Profile vs. Practicality
Transdermal options offer a clear safety advantage regarding blood clot risk. The primary trade-offs are practical: some individuals may experience skin irritation from patches, and gels require careful daily application to ensure proper absorption.
Making the Right Choice for Your Health
Your decision should be based on a comprehensive evaluation of your personal health history and priorities, made in partnership with your healthcare provider.
- If your primary focus is bone health or you're weighing breast cancer risk: The choice of delivery method is not the deciding factor for these specific outcomes.
- If you have any risk factors for blood clots, stroke, or liver disease: Transdermal therapy is generally the safer first-line option, as it avoids the first-pass effect in the liver.
- If your priority is convenience and you have a low cardiovascular risk profile: Oral therapy is a highly effective option that may be perfectly appropriate for you.
Ultimately, the best hormone therapy plan is one that is tailored specifically to your individual physiology and health goals.
Summary Table:
| Feature | Oral Therapy | Transdermal Therapy |
|---|---|---|
| Fracture Risk Reduction | Similar systemic effect | Similar systemic effect |
| Breast Cancer Risk Impact | Comparable at same dose | Comparable at same dose |
| Blood Clot (VTE) Risk | Higher due to first-pass liver metabolism | Lower, bypasses liver |
| Primary Consideration | Convenience; suitable for low cardiovascular risk | Safer for individuals with clotting risk factors |
Partner with Enokon for Your Hormone Therapy Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with high-quality, consistent hormone delivery systems. Our technical expertise ensures optimal product performance and patient safety.
Leverage our custom R&D and development services to create tailored transdermal solutions that meet specific therapeutic requirements and enhance patient outcomes.
Contact our experts today to discuss how we can support your product development and supply chain.
Visual Guide
Related Products
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Prostate Pain Kidney Health Care Patch for Men
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- What are the serious side effects of menthol patch that require immediate medical attention?
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- What is the primary use of a menthol patch? Targeted Relief for Muscle & Joint Pain
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief














