At its core, the buprenorphine transdermal patch is a sophisticated delivery system. It works by continuously releasing a powerful opioid pain reliever, buprenorphine, from an adhesive matrix directly through your skin and into your bloodstream. This provides a steady dose of medication that alters how your brain and nervous system perceive severe, chronic pain.
The buprenorphine patch is designed for one specific purpose: to deliver a slow, consistent, and long-lasting dose of opioid medication for severe pain that requires around-the-clock treatment, bypassing the digestive system entirely.
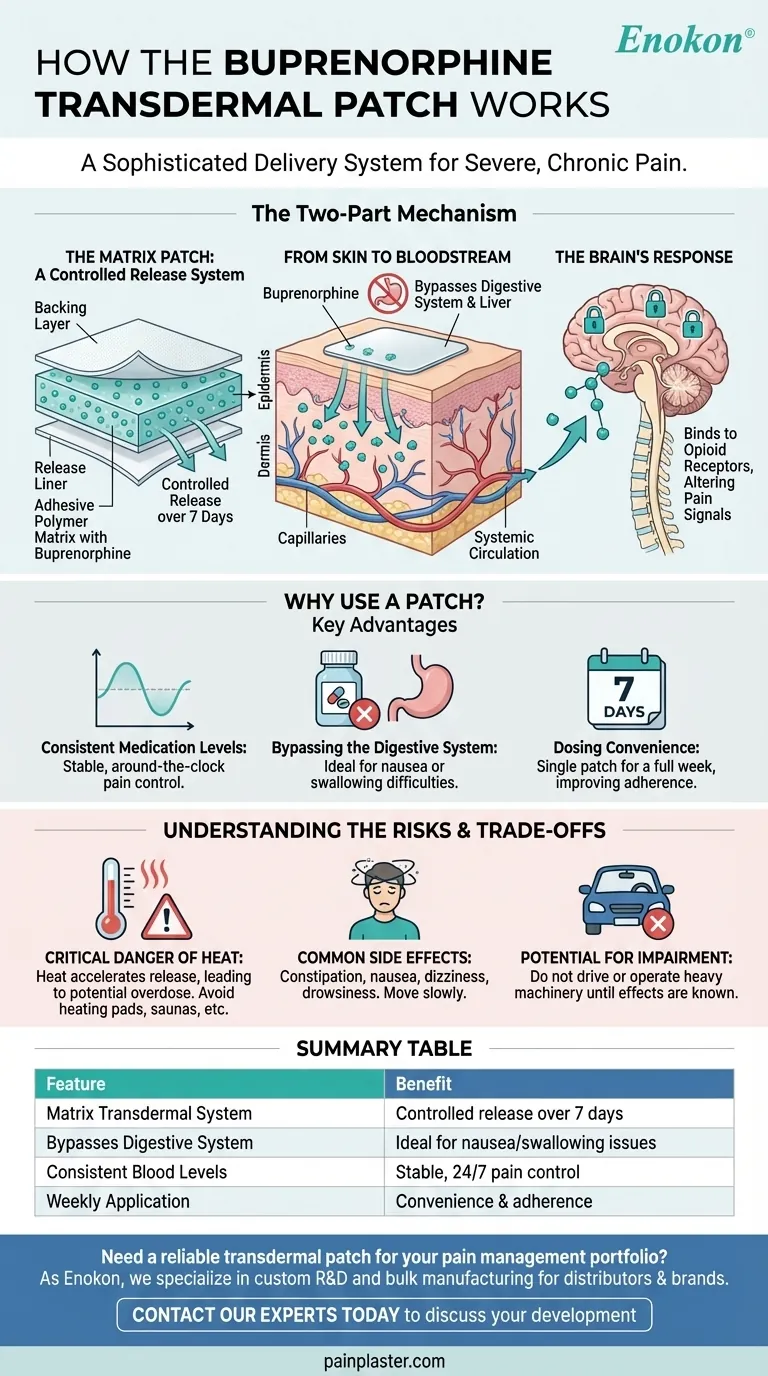
The Two-Part Mechanism: From Patch to Pain Relief
Understanding how the patch works requires looking at two distinct components: the physical patch technology and the pharmacological action of the drug itself.
The Matrix Patch: A Controlled Release System
The patch is not simply a medicated bandage. It is a matrix transdermal system.
This means the buprenorphine is evenly mixed into an adhesive polymer layer. This design ensures a gradual and controlled release of the drug over several days.
The rate of delivery is precisely engineered into the patch's formulation, minimizing the risk of accidental, large-dose releases.
From Skin to Bloodstream
Once applied, the buprenorphine begins to diffuse from the patch's matrix through the outer layers of your skin.
It eventually reaches the network of tiny blood vessels (capillaries) in the deeper skin layers, where it enters your systemic circulation.
This transdermal route allows the medication to be distributed throughout your body without first being processed by your liver, a key difference from oral medications.
The Brain's Response: Altering Pain Signals
After entering the bloodstream, buprenorphine travels to the central nervous system.
As an opiate analgesic, it binds to specific opioid receptors in the brain and spinal cord.
This action mimics the effect of natural pain-relieving chemicals, effectively changing your body's response to pain signals and reducing the sensation of pain.
Why Use a Patch? Key Advantages
The transdermal patch is prescribed for severe, persistent pain when other options are inadequate. Its unique delivery method offers specific benefits.
Consistent Medication Levels
Unlike pills, which can create peaks and valleys in medication levels, the patch provides a consistent, steady state of buprenorphine in the blood.
This can result in more stable, around-the-clock pain control without the ups and downs of intermittent dosing.
Bypassing the Digestive System
The patch is an ideal solution for patients who have difficulty swallowing pills or experience severe nausea and vomiting.
Because the drug is absorbed through the skin, it completely avoids the gastrointestinal tract.
Dosing Convenience
A single patch is typically worn for seven days, offering a significant convenience over medications that must be taken multiple times a day.
This simplified regimen can improve treatment adherence and overall quality of life.
Understanding the Risks and Trade-offs
While effective, the buprenorphine patch carries significant risks that demand careful management and full awareness.
The Critical Danger of Heat
Exposing the patch to heat from sources like heating pads, electric blankets, saunas, or even a high fever can be extremely dangerous.
Heat accelerates the rate of drug release, which can cause a sudden, large amount of buprenorphine to enter your bloodstream, leading to a potential overdose.
Common Side Effects
Like all opioids, buprenorphine can cause side effects. These often include constipation, nausea, dizziness, drowsiness, and headache.
Dizziness upon standing up quickly is a common issue, requiring you to move slowly and carefully.
Potential for Impairment
This medication can significantly impair your judgment and reaction time.
You must not drive or operate heavy machinery until you understand exactly how the patch affects you. Combining it with alcohol or other sedating drugs dramatically increases this risk.
Making the Right Choice for Your Pain Management
The decision to use a buprenorphine patch is a serious one, made between you and your healthcare provider based on the specific nature of your pain.
- If your primary focus is managing severe, around-the-clock pain: The patch provides a powerful and consistent solution specifically designed for persistent pain that has not responded to other treatments.
- If your primary focus is convenience and avoiding pills: The once-weekly application simplifies treatment, especially for individuals with chronic nausea or difficulty swallowing.
- If your primary focus is safety: You must be absolutely committed to avoiding all external heat sources on the patch and monitoring closely for side effects with your doctor.
Ultimately, understanding how this technology works empowers you to use it safely and effectively as part of a comprehensive pain management plan.
Summary Table:
| Feature | Benefit |
|---|---|
| Matrix Transdermal System | Ensures a slow, controlled release of medication over 7 days. |
| Bypasses Digestive System | Ideal for patients with nausea or difficulty swallowing pills. |
| Consistent Blood Levels | Provides stable, around-the-clock pain control. |
| Weekly Application | Offers significant convenience and improved treatment adherence. |
Need a reliable transdermal patch for your pain management portfolio?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we specialize in custom R&D to develop solutions tailored for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise to create a product that meets your specific market needs.
Contact our experts today to discuss your custom transdermal patch development.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the withdrawal symptoms if transdermal buprenorphine is stopped suddenly? Avoid Severe Discomfort with a Safe Tapering Plan
- What storage conditions are required for methylphenidate transdermal? Ensure Patch Stability & Safety
- What are the dosage options for the patch? A Guide to 6mg, 9mg, and 12mg Strengths
- What role do Fentanyl Transdermal Patches play in cancer pain? Explore Stable, 72-Hour Analgesia Delivery
- What precautions should be taken regarding allergies before using selegiline transdermal patch? Ensure Safe Application and Avoid Reactions
- Why are peel adhesion and rolling ball tack tests necessary? Ensuring Reliable Transdermal Patch Performance
- How should the topical diclofenac patch or system be applied? A Step-by-Step Guide for Safe, Effective Pain Relief
- What is the role of medical-grade placebo patches in transdermal drug R&D? Secure Reliable Clinical Trial Data
















