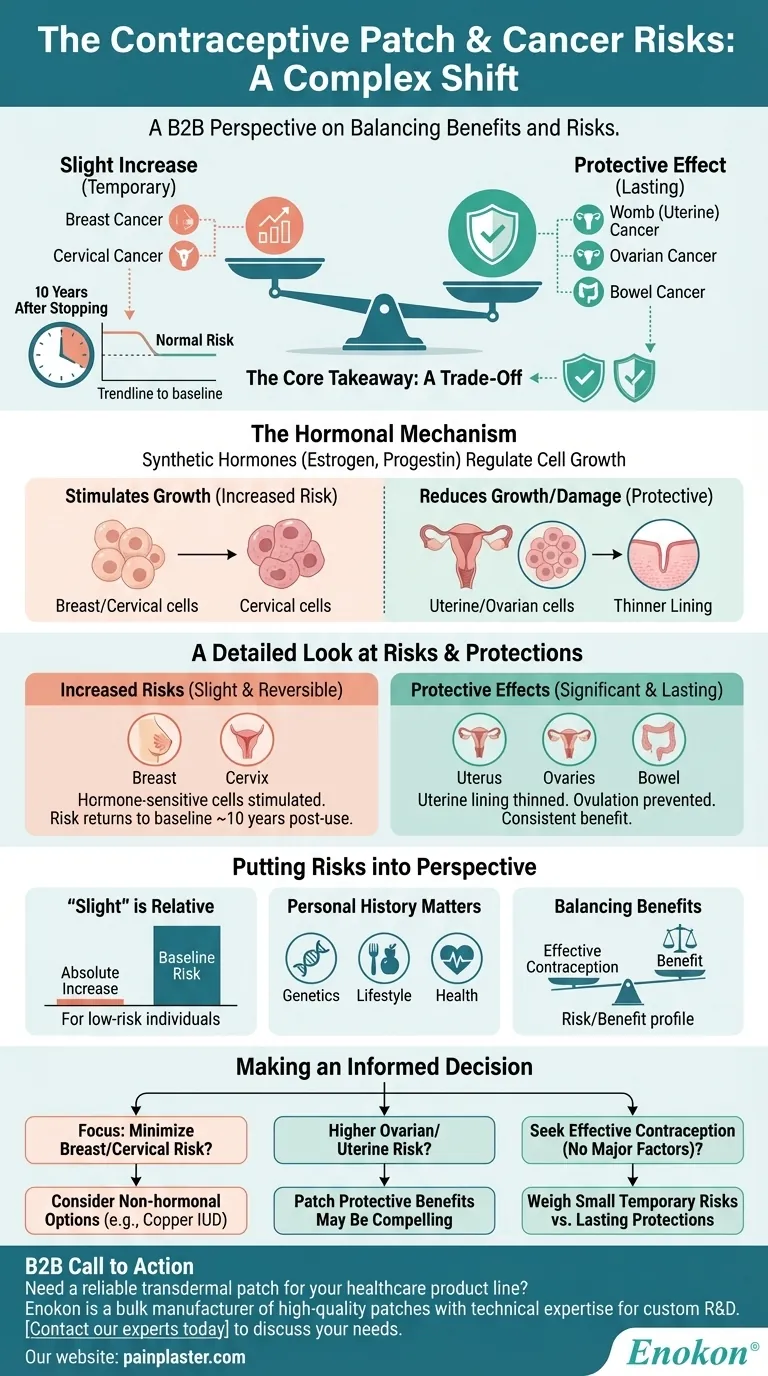
Using the contraceptive patch involves a complex shift in cancer risks, not a simple increase or decrease. It slightly raises the risk for breast and cervical cancer, but this elevated risk diminishes and returns to normal about 10 years after you stop using it. Conversely, it provides a notable protective effect by lowering the risk of developing ovarian, womb (uterine), and bowel cancers.
The core takeaway is that the contraceptive patch creates a trade-off. It introduces a small, temporary increase in the risk for certain hormone-sensitive cancers while simultaneously offering a lasting protective benefit against others.
The Hormonal Mechanism: Why the Patch Influences Risk
How Hormones Regulate Cell Growth
The contraceptive patch works by releasing synthetic versions of the hormones estrogen and progestin into your body. These hormones prevent pregnancy primarily by stopping ovulation.
A secondary effect of these hormones is their influence on cell division in different parts of the body. This ability to regulate cell growth is the fundamental reason the patch can both increase and decrease the risk of different types of cancers.
A Detailed Look at the Increased Risks
Breast Cancer Risk
Use of the patch is associated with a slight increase in the risk of breast cancer. This is because some breast cells are sensitive to the hormones released by the patch, which can stimulate their growth.
Cervical Cancer Risk
Similarly, there is a slight increase in the risk of cervical cancer. The exact mechanism is still being studied, but it is believed to be related to how hormonal changes may affect the body's susceptibility to HPV, the primary cause of cervical cancer.
The Reversibility Factor
A critical piece of context is that these increased risks are not permanent. The evidence shows that about 10 years after discontinuing the patch, your risk for both breast and cervical cancer returns to the baseline level of someone who has never used it.
Understanding the Protective Effects
Womb (Uterine) Cancer Protection
The patch has a significant protective effect, lowering the risk of developing cancer of the womb (also known as endometrial or uterine cancer). The progestin in the patch helps to thin the uterine lining, which reduces the cell growth that can lead to cancer.
Ovarian Cancer Protection
Users also see a reduced risk of ovarian cancer. By preventing ovulation, the patch reduces the cellular changes and damage on the surface of the ovaries that can occur with each monthly cycle, a key factor in cancer development.
Bowel Cancer Protection
The patch also appears to lower the risk of bowel (colorectal) cancer. While the exact reasons are less clear than for uterine or ovarian cancer, it is a consistent and beneficial finding associated with combined hormonal contraceptives.
Putting the Risks into Perspective
"Slight" is a Key Term
It is essential to understand that a "slight" increase in risk is a relative term. For individuals with a low baseline risk of breast or cervical cancer, the absolute increase in risk remains very small.
Balancing Benefits and Risks
The decision to use the patch involves weighing its primary purpose—effective contraception—against this complex profile of risks and benefits. For many, the protective effects against three other cancers are a significant factor in this calculation.
The Importance of Personal History
Your individual risk profile is unique. Factors like your family medical history, lifestyle choices, and personal health are crucial. These risks do not exist in a vacuum and should be discussed with a healthcare provider who understands your full medical context.
Making an Informed Decision
To make the right choice, you must align the patch's risk profile with your personal health priorities and concerns.
- If your primary focus is minimizing breast or cervical cancer risk: Discussing non-hormonal contraceptive options, like the copper IUD, with your doctor would be a logical next step.
- If you have a higher personal or family risk of ovarian or uterine cancer: The significant protective benefits of the patch in these areas might make it a compelling choice for you.
- If you are seeking effective contraception without major pre-existing risk factors: The key is to weigh the small, temporary increase in some risks against the notable and lasting decrease in others.
Ultimately, a conversation with your healthcare provider is the best way to make a decision that is medically sound and right for you.
Summary Table:
| Cancer Type | Effect of Contraceptive Patch | Key Details |
|---|---|---|
| Breast Cancer | Slight Increase | Risk returns to normal ~10 years after stopping use. |
| Cervical Cancer | Slight Increase | Risk returns to normal ~10 years after stopping use. |
| Ovarian Cancer | Decreased Risk | Protective effect from preventing ovulation. |
| Womb (Uterine) Cancer | Decreased Risk | Progestin thins uterine lining, reducing risk. |
| Bowel Cancer | Decreased Risk | Consistent protective benefit observed. |
Need a reliable transdermal patch for your healthcare product line?
As a bulk manufacturer of high-quality transdermal patches, Enokon provides pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Whether you're launching a new contraceptive patch or another topical solution, we ensure reliability and efficacy.
Contact our experts today to discuss how we can support your product development needs.
Visual Guide
Related Products
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- Can heating pads be used with pain patches? Risks & Safety Tips
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How often should HRT patches be changed? Optimize Your Hormone Therapy Schedule
- Why should heating pads not be used with transdermal patches? Avoid Overdose & Skin Risks














