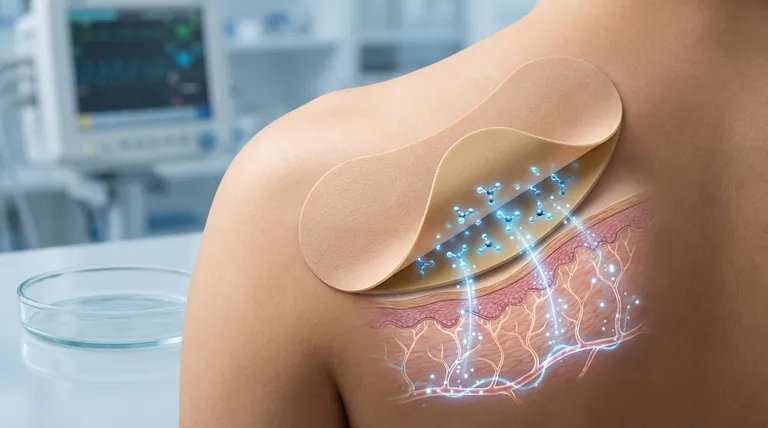
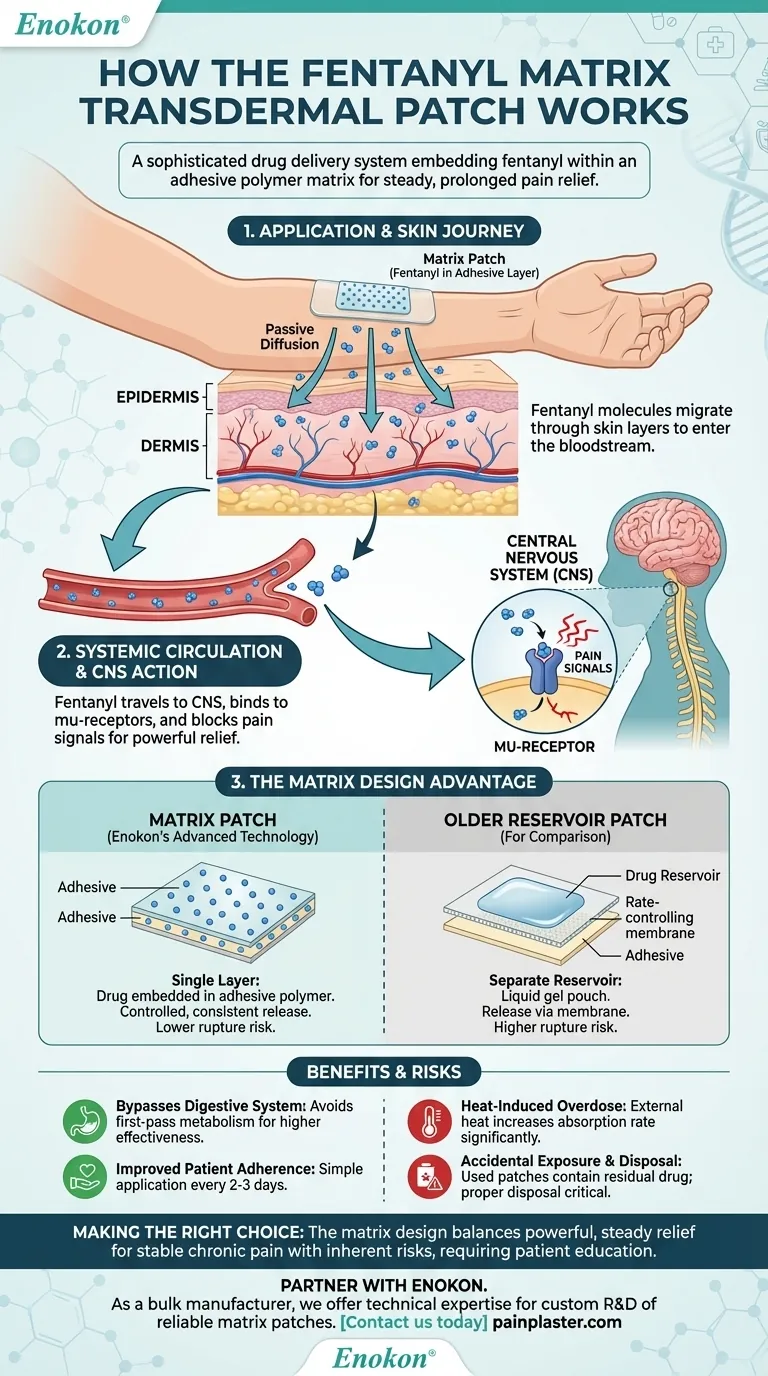
At its core, a fentanyl matrix transdermal patch is a sophisticated drug delivery system designed to administer a potent opioid through the skin over a prolonged period. The "matrix" design embeds fentanyl directly within an adhesive polymer layer, which is then applied to the skin. From there, the drug gradually diffuses through the skin layers to enter the bloodstream, providing steady, long-term pain relief.
The key innovation of the matrix patch is its structure. By mixing the drug directly into the adhesive, it creates a more stable and controlled system that minimizes the risk of accidental overdose from a ruptured patch, a significant concern with older reservoir-style designs.
The Mechanism: From Skin to Nervous System
The fentanyl patch operates on the principle of passive diffusion, moving medication from an area of high concentration (the patch) to an area of low concentration (the body). This process bypasses the digestive system entirely.
The Journey Through the Skin
A transdermal patch consists of several layers, including a protective backing and the critical drug-in-adhesive matrix. Once applied, fentanyl molecules begin to migrate from this matrix, pass through the outer layer of the skin (the epidermis), and reach the deeper layer (the dermis).
The dermis is rich in blood vessels. As fentanyl reaches this layer, it is absorbed into the systemic circulation, allowing it to be distributed throughout the body.
Action in the Central Nervous System
Once in the bloodstream, fentanyl travels to the central nervous system (CNS). There, it selectively binds to mu-receptors, which are the same receptors that the body's natural pain-relieving chemicals (endogenous opiates) use.
By activating these receptors, fentanyl effectively blocks pain signals from reaching the brain, resulting in powerful pain relief.
The "Matrix" Design: A Critical Distinction
Not all transdermal patches are built the same. The matrix design represents a significant advancement in safety and consistency over older "reservoir" patches, which held the drug in a liquid gel pouch.
A Single, Unified Layer
In a matrix patch, there is no separate drug reservoir. The fentanyl is evenly and molecularly distributed throughout the adhesive polymer matrix itself. This unified layer serves as both the drug source and the means of sticking to the skin.
Controlled and Consistent Release
The rate at which fentanyl is released is controlled by the specific formulation of the polymer matrix and the concentration gradient between the patch and the skin. This design ensures a slow, predictable, and continuous release of medication over 2-3 days.
This steady delivery avoids the peaks and troughs in blood concentration often seen with oral medications, leading to more consistent therapeutic outcomes for chronic pain management.
Understanding the Trade-offs and Benefits
The matrix patch offers clear advantages but also comes with important considerations that require careful management.
Benefit: Bypassing the Digestive System
By delivering the drug through the skin, the patch avoids first-pass metabolism. This is a process where orally ingested drugs are broken down by the liver before they can circulate through the body, often reducing their effectiveness. Transdermal delivery ensures more of the drug reaches its target.
Benefit: Improved Patient Adherence
For patients with chronic pain, applying a patch every few days is far simpler than remembering to take multiple pills each day. This can significantly improve adherence to a prescribed pain management regimen.
Risk: Heat-Induced Overdose
Exposing the patch to external heat sources—such as heating pads, electric blankets, or even a high fever—can increase the rate of drug absorption significantly. This can lead to a dangerously high level of fentanyl in the blood, potentially causing a life-threatening overdose.
Risk: Accidental Exposure and Disposal
The adhesive matrix still contains a significant amount of fentanyl even after it has been worn. Improper disposal can lead to accidental exposure for children, pets, or other adults, which can be fatal. It is critical to follow specific disposal instructions, such as folding the patch in half so the adhesive side sticks to itself.
Making the Right Choice for Pain Management
Understanding the mechanics of the fentanyl matrix patch is essential for its safe and effective clinical use. The choice to use it is based on balancing its powerful, steady relief against its inherent risks.
- If the primary goal is managing stable, chronic pain: The matrix patch is an excellent choice for providing consistent, around-the-clock analgesia without the peaks and valleys of short-acting opioids.
- If the patient has difficulty with oral medications: The patch is a crucial alternative for individuals who cannot swallow pills or who experience severe gastrointestinal side effects from oral opioids.
- If safety is the paramount concern: The matrix design is inherently safer than older reservoir patches, but patient education on heat avoidance and proper disposal is non-negotiable to prevent accidental overdose.
Ultimately, mastering this technology depends on respecting both its therapeutic power and its potential for harm.
Summary Table:
| Aspect | Matrix Patch | Reservoir Patch |
|---|---|---|
| Design | Drug is embedded in the adhesive layer. | Drug is held in a separate gel pouch. |
| Safety | Lower risk of accidental overdose if damaged. | Higher risk if the reservoir ruptures. |
| Release | Controlled, steady release via the polymer matrix. | Release controlled by a membrane. |
Need a reliable partner for your transdermal patch development?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Benefit from our advanced matrix technology to create safe, effective, and consistent pain management solutions for your patients.
Contact our experts today to discuss your custom patch requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How should the patch be stored? Essential Tips for Preserving Herbal Patch Efficacy
- What should be done if a Butrans patch comes into contact with hands? Immediate Steps for a Potential Overdose
- What are the potential side effects of buprenorphine patches? Risks & Safety Tips
- What are the contraindications for transdermal fentanyl? Safely Navigating Its Strict Use for Chronic Pain
- What is the impact of external heat sources on Asenapine transdermal patches? Avoid Dangerous Dose Dumping Risks
- What storage conditions are required for methylphenidate transdermal? Ensure Patch Stability & Safety
- What should be done if the scopolamine transdermal patch falls off? Quick Fixes & Prevention Tips
- How long can the essential oil patch be worn? Get 24 Hours of Consistent Relief Safely
















