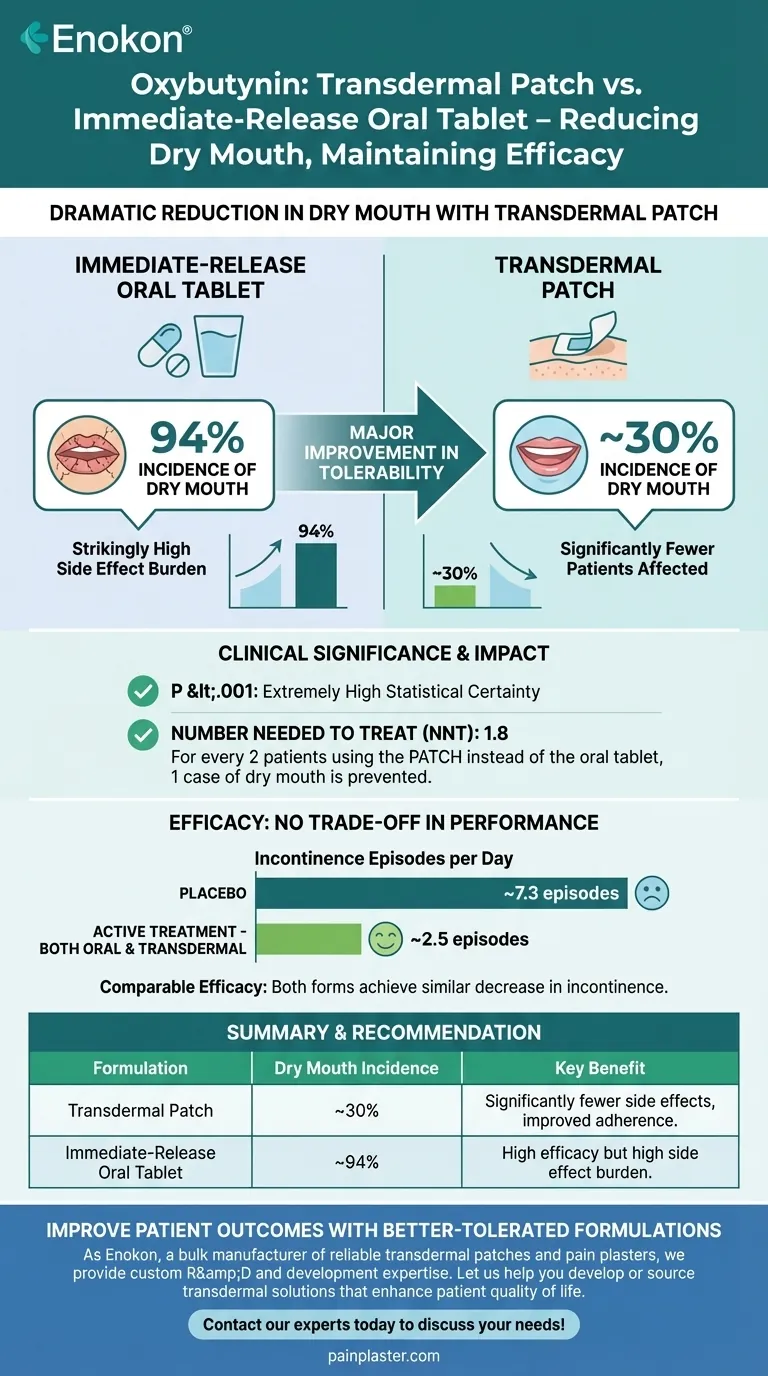
Notably, the transdermal form of oxybutynin causes dry mouth in significantly fewer patients than the immediate-release oral form. Based on clinical data, the incidence of dry mouth is approximately 30 percent with the transdermal patch, compared to a striking 94 percent with the immediate-release oral tablet. This difference is not only statistically significant but also represents a major improvement in the drug's tolerability.
The core takeaway is that switching from oral to transdermal oxybutynin can dramatically reduce the risk of dry mouth without compromising the medication's effectiveness in controlling incontinence episodes.
The Stark Contrast in Side Effects
When evaluating different drug formulations, the side effect profile is as critical as the therapeutic benefit. For oxybutynin, the delivery method is the primary determinant of its most common side effect.
Quantifying the Difference
In a direct comparison study, the numbers are unambiguous. 94 percent of patients taking immediate-release oral oxybutynin reported experiencing dry mouth.
In contrast, only 30 percent of patients using the transdermal patch reported the same side effect.
Clinical and Statistical Significance
This finding carries a high degree of statistical certainty (P <.001), indicating the result is extremely unlikely to be due to random chance.
The Number Needed to Treat (NNT) is 1.8. This powerful metric means that for every two patients who use the transdermal patch instead of the oral tablet, one case of dry mouth is prevented.
Efficacy: Is There a Performance Trade-off?
A common concern when changing a medication's delivery system is whether its effectiveness will be diminished. The evidence shows this is not the case for oxybutynin.
Equivalent Incontinence Control
A study of patients who had previously responded well to oral oxybutynin demonstrated the transdermal patch's comparable efficacy.
Both the oral and transdermal forms produced a similar decrease in the number of daily incontinence episodes.
Measuring the Therapeutic Outcome
Patients in the study went from approximately 7.3 incontinence episodes per day on placebo down to roughly 2.5 episodes per day while on active treatment.
Crucially, this excellent therapeutic outcome was achieved regardless of whether the patient was using the oral or the transdermal formulation. This shows that the improved side effect profile does not come at the cost of performance.
Making the Right Choice for Your Goal
The data provides a clear path for choosing the optimal formulation based on treatment priorities.
- If your primary focus is minimizing side effects and improving adherence: The transdermal patch is the superior choice, as it dramatically reduces the risk of dry mouth.
- If your primary focus is effective incontinence control: Both the transdermal and oral forms are clinically effective, but the transdermal patch achieves this goal with a much lower burden of side effects.
Ultimately, the transdermal delivery system offers a way to achieve the desired therapeutic benefit of oxybutynin while significantly improving the patient's quality of life.
Summary Table:
| Formulation | Incidence of Dry Mouth | Key Benefit |
|---|---|---|
| Transdermal Patch | ~30% | Significantly fewer side effects, improved patient adherence |
| Immediate-Release Oral Tablet | ~94% | High efficacy but high side effect burden |
Ready to improve patient outcomes with better-tolerated oxybutynin formulations? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with custom R&D and development expertise. Let us help you develop or source transdermal solutions that enhance patient quality of life. Contact our experts today to discuss your needs!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What role do Franz diffusion cells play in evaluating transdermal penetration performance? Optimize Your Drug Delivery
- How can patch administration be halted? Take Instant Control of Your Medication Delivery
- What were the findings from the patient satisfaction survey regarding transdermal systems? High Preference, Ease of Use
- What is the summary of key points about Rivastigmine patches? Key Benefits & Considerations
- What information can Coarse-Grained Molecular Dynamics (CG-MD) provide? Reveal Hidden Mechanisms in Transdermal Research
- How do statistical analysis and mathematical modeling assist in transdermal drug optimization? Data-Driven Solutions
- What is the role of solvent partition extraction in Siegesbeckia herba extracts? Optimize Your Skincare Formulations
- What are the interactions of testosterone transdermal patch with other medicines? Key Risks & Safety Tips














