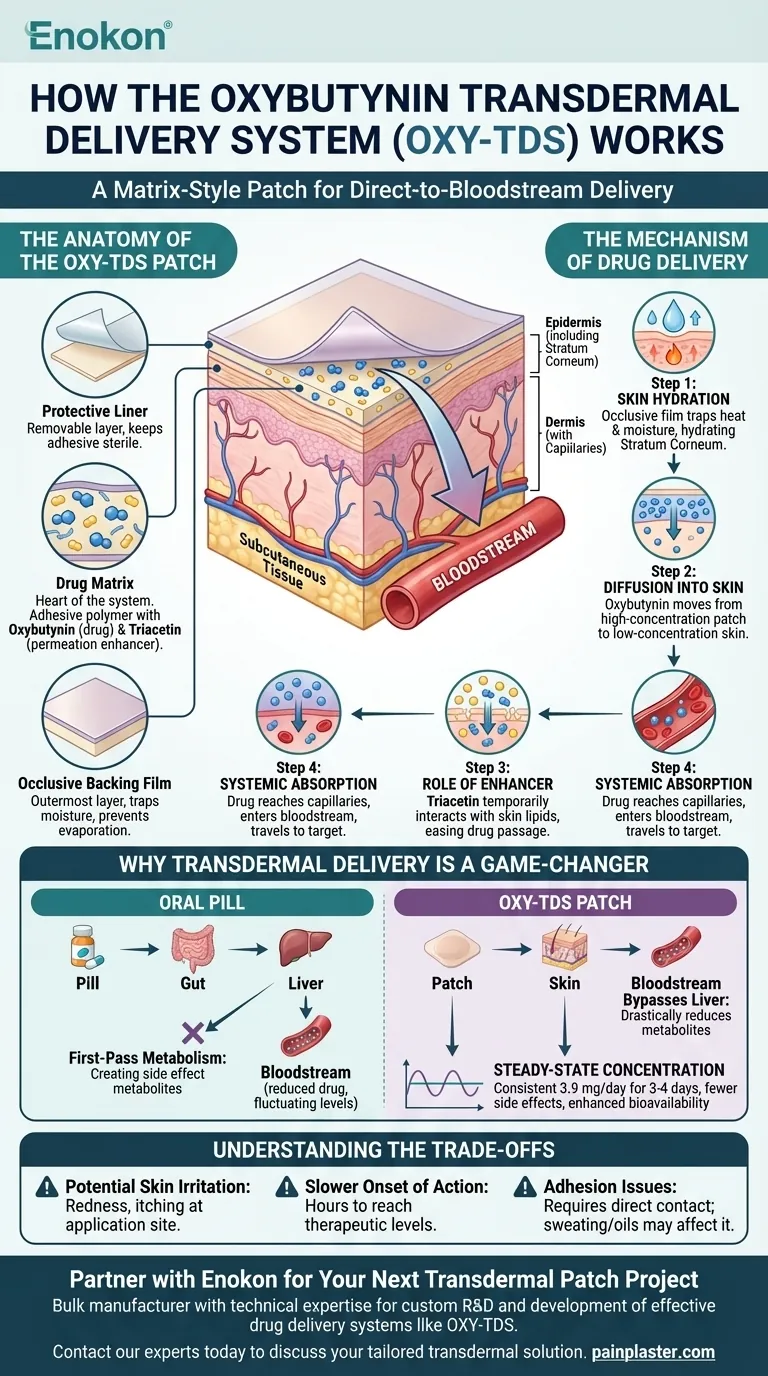
At its core, the oxybutynin transdermal delivery system (OXY-TDS) is a matrix-style patch that delivers the medication directly through the skin into the bloodstream. It consists of an adhesive polymer matrix that contains the drug (oxybutynin) and a permeation enhancer (triacetin). This design allows for a slow, continuous release of the drug over several days, bypassing the digestive system entirely.
The fundamental advantage of the OXY-TDS patch is its ability to avoid the liver's "first-pass metabolism." This mechanism delivers a steady, controlled dose of oxybutynin, which significantly reduces the severe side effects, like dry mouth, commonly associated with the oral pill form of the medication.

The Anatomy of the OXY-TDS Patch
The patch is a sophisticated, multi-layered system designed for controlled drug release. Each component serves a specific and critical function.
The Protective Liner
This is the layer you peel off just before application. Its sole purpose is to protect the drug-infused adhesive and keep it sterile until it's placed on the skin.
The Drug Matrix
This is the heart of the system. It's an adhesive polymer layer where the active drug, oxybutynin, is evenly distributed. This layer also contains triacetin, a crucial permeation enhancer that helps the drug penetrate the skin.
The Occlusive Backing Film
The outermost layer of the patch is the backing film. It is "occlusive," meaning it prevents the drug from evaporating and traps moisture underneath, which aids in the absorption process.
The Mechanism of Drug Delivery
Getting a drug from a patch into your bloodstream is a multi-step process driven by simple physics and clever chemistry.
Step 1: Skin Hydration
Once applied, the occlusive backing film traps the natural moisture and heat from your skin. This hydration of the outermost skin layer, the stratum corneum, makes it more permeable and receptive to the drug.
Step 2: Diffusion into the Skin
The drug is highly concentrated in the patch's matrix. This creates a concentration gradient, causing the oxybutynin molecules to naturally move from the high-concentration patch into the low-concentration environment of your skin.
Step 3: The Role of the Permeation Enhancer
This is where the chemical engineering shines. The enhancer, triacetin, diffuses into the skin alongside the drug. It temporarily and reversibly interacts with the natural lipids (fats) in the skin, making it easier for the oxybutynin molecules to pass through this protective barrier.
Step 4: Systemic Absorption
Once past the stratum corneum, the oxybutynin enters the deeper layers of the skin and is absorbed by the dense network of capillaries. From there, it enters the systemic circulation—the bloodstream—and travels throughout the body to its target destination.
Why Transdermal Delivery is a Game-Changer
The choice to use a patch instead of a pill for oxybutynin is not arbitrary; it solves the primary problems associated with the oral version of the drug.
Bypassing First-Pass Metabolism
When you swallow an oxybutynin pill, it is absorbed by the gut and sent directly to the liver. The liver extensively metabolizes it, creating a large amount of a metabolite that is a primary cause of side effects like severe dry mouth and constipation. By entering the bloodstream through the skin, the patch bypasses the liver almost entirely, drastically reducing the formation of these problematic metabolites.
Achieving Steady-State Concentration
Oral pills create a "peak and trough" effect, where drug levels spike after taking a pill and fall before the next dose. The OXY-TDS patch delivers a consistent 3.9 mg of oxybutynin per day for its entire application period (typically 3-4 days). This steady level provides more consistent symptom control with fewer side effects.
Enhanced Bioavailability
Because the drug isn't broken down by the digestive system or liver, a much higher percentage of the active medication reaches the bloodstream. This makes the delivery method highly efficient and predictable.
Understanding the Trade-offs
While highly effective, the transdermal system is not without its own set of considerations.
Potential for Skin Irritation
The most common side effect of the OXY-TDS patch is localized skin reaction. Redness, itching, or a rash can occur at the application site due to the adhesive or the drug itself.
Slower Onset of Action
Unlike a pill, a patch does not provide immediate effects. It takes several hours for the drug to penetrate the skin and reach therapeutic levels in the bloodstream, making it unsuitable for acute symptom relief.
Adhesion Issues
The patch must maintain direct contact with the skin to work correctly. Excessive sweating, oils, or improper application can cause the patch to loosen or fall off, interrupting drug delivery.
Making the Right Choice for Your Goal
Understanding the delivery mechanism helps you and your healthcare provider determine if this is the best approach for your specific needs.
- If your primary focus is minimizing side effects: The OXY-TDS patch is often a superior choice, as it is specifically designed to avoid the metabolic process that causes the most common oral side effects.
- If your primary focus is convenience and stable symptom control: The twice-weekly application provides a steady, reliable dose of medication without the need to remember daily pills.
- If you have very sensitive skin or need immediate relief: You should discuss alternatives with your provider, as the patch may cause local irritation and has a delayed onset of action.
By delivering medication through the skin, the OXY-TDS patch leverages a unique pathway to maximize therapeutic benefits while minimizing systemic drawbacks.
Summary Table:
| Key Component | Function |
|---|---|
| Drug Matrix | Adhesive layer containing oxybutynin and permeation enhancer (Triacetin). |
| Occlusive Backing Film | Traps skin moisture to aid drug absorption and prevents evaporation. |
| Protective Liner | Removable layer that keeps the adhesive sterile before application. |
| Delivery Mechanism | Drug diffuses through skin into capillaries, bypassing the digestive system. |
| Primary Benefit | Avoids first-pass liver metabolism, significantly reducing systemic side effects. |
Partner with Enokon for Your Next Transdermal Patch Project
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Benefit from our experience in creating sophisticated, effective drug delivery systems like the OXY-TDS.
Contact our experts today to discuss how we can develop a transdermal solution tailored to your needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What precautions should be taken while using selegiline skin patch? Ensure Safe & Effective Treatment
- Why are industrial-grade dialysis bags required for silk fibroin extraction? Ensuring Purity for Transdermal Products
- What are the modern enhancement methods for transdermal drug delivery? Breakthroughs in Skin Permeation Technology
- How should nicotine patches be applied for optimal use? Master the Correct Technique for Maximum Relief
- What lifecycle management benefits do patches provide? A Strategic Platform for Long-Term Product Success
- What are the risks associated with the birth control patch? Understand the Blood Clot & Estrogen Dangers
- What are the side effects or health risks of the birth control patch? Understanding the Risks and Benefits
- How is the estradiol transdermal patch metabolized and eliminated? Understanding the Metabolic Pathway














