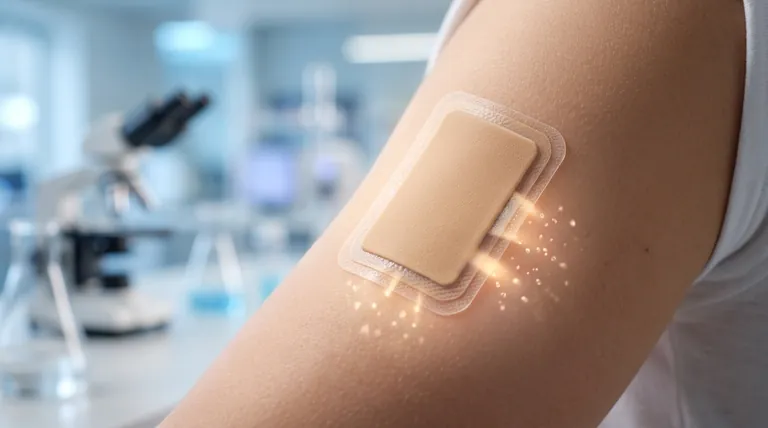
At its core, the clonidine transdermal system is a passive device. It releases the medication at a constant, pre-programmed rate over seven days. The entire process is powered by the natural principle of a concentration gradient—the difference between the high concentration of clonidine saturated in the patch and the much lower concentration in the skin.
The clonidine patch leverages a simple physical principle—diffusion—to transform a pill's fluctuating dosage into a stable, week-long therapeutic effect. The key is understanding that this stability comes with a significant time lag for both the onset of action and the drug's departure from the body.
The Core Mechanism: Passive Diffusion
The transdermal system is not a complex electronic pump; it is a sophisticated bandage designed for predictable, passive drug delivery. Its function is governed by a few key principles.
The Concentration Gradient as the Engine
The driving force for the drug's release is the concentration gradient. The patch contains a saturated solution of clonidine, creating a high-concentration reservoir. Your skin, in contrast, has a very low concentration. This difference creates a natural "pressure" that pushes the clonidine molecules out of the patch and through the outer layers of your skin into the bloodstream.
Achieving a Constant Rate
The system is engineered to maintain this concentration gradient at a steady level for a full week. By keeping the drug solution saturated, the patch ensures that the "push" of clonidine into the skin remains constant and predictable, avoiding the peaks and valleys associated with oral medications.
The Role of Patch Size
The total daily dose is controlled by a simple, direct relationship: the surface area of the patch. A larger patch has more surface area in contact with the skin, allowing more drug molecules to pass through per day. The release is directly proportional to this area:
- A 3.5 cm² patch delivers 0.1 mg/day.
- A 7.0 cm² patch delivers 0.2 mg/day.
- A 10.5 cm² patch delivers 0.3 mg/day.
Clinical Implications of This Release System
This unique delivery method has significant consequences for how the drug behaves in the body and how it should be managed clinically.
Time to Therapeutic Effect
Because the release is slow and steady, it takes two to three days for the clonidine concentration in the blood to reach a stable, therapeutic level. This delay is a critical factor; the patch is not suitable for acute or immediate blood pressure control.
Steady-State Plasma Levels
Once therapeutic levels are achieved, they remain remarkably stable. This "steady-state" is the primary advantage of the transdermal system, providing consistent blood pressure control and potentially reducing side effects that can occur when drug levels spike.
The "Reservoir" Effect After Removal
After the patch is removed, the clonidine that has already been absorbed into the upper layers of the skin continues to be released into the bloodstream. This creates a skin "reservoir" that causes the drug's concentration to decline slowly, with a half-life of approximately 20 hours.
Understanding the Trade-offs and Precautions
While effective, this delivery system has inherent trade-offs and requires specific safety considerations.
Delayed Onset and Offset
The slow diffusion process is a double-edged sword. It provides stability but also means the therapeutic effect is slow to start and slow to stop. Discontinuing therapy requires planning and monitoring, as the drug's effects will linger for more than a day after the patch is removed.
Interaction with Medical Procedures
The patch contains an aluminum backing layer. This is a critical safety detail. The patch must be removed before an MRI, defibrillation, or cardioversion to prevent the aluminum from conducting electricity and causing skin burns.
Considerations for Renal Impairment
A significant portion of the absorbed clonidine (40-60%) is excreted unchanged by the kidneys. Therefore, patients with renal impairment require careful monitoring, as their ability to clear the drug is reduced, potentially leading to accumulation.
Making the Right Choice for Your Goal
- If your primary focus is stable, long-term blood pressure control: The transdermal system is an excellent choice for its ability to provide consistent drug levels and avoid the fluctuations of oral dosing.
- If your primary focus is rapid blood pressure reduction: This system is inappropriate due to the two-to-three-day delay in reaching therapeutic effect.
- If a patient is scheduled for an MRI or cardioversion: It is critical to remember to remove the patch beforehand to prevent skin burns from the aluminum backing.
- If you are discontinuing therapy: You must account for the long half-life and "reservoir" effect, monitoring the patient for several days after patch removal.
Understanding the passive, gradient-driven release of the clonidine patch is the key to leveraging its benefits for stable therapy while safely navigating its inherent limitations.
Summary Table:
| Key Characteristic | Details |
|---|---|
| Mechanism | Passive diffusion driven by a concentration gradient |
| Duration | Consistent release over 7 days |
| Dosage Control | Directly proportional to patch surface area |
| Time to Steady State | 2-3 days to reach therapeutic effect |
| Half-Life After Removal | ~20 hours (due to skin reservoir effect) |
Need a reliable transdermal delivery system for your pharmaceutical product?
At Enokon, we are a bulk manufacturer of high-quality, reliable transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to bring their products to market.
Leverage our technical expertise for custom R&D and development to create a delivery system that meets your specific therapeutic goals, just like the clonidine patch.
Contact our experts today to discuss your project and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- What is the significance of TEM in evaluating green tea transfersomes? Expert Insights on Nanoscale Morphology
- What should be done if you forget to take the patch off? Patch Replacement Guidelines
- How can the risk of serious health problems be minimized while using transdermal estradiol? A Guide to Safe Hormone Therapy
- Can the birth control patch affect menstrual cycles? Understanding the expected changes.
- What should be done if the birth control patch is forgotten or falls off? A Guide to Staying Protected
- How do transdermal patches simplify treatment regimens? Reduce Pill Burden with Continuous Delivery
- How should used granisetron patches be disposed of? Ensure Safe & Compliant Disposal
- What is a nicotine patch and how does it work? A Guide to Quitting Smoking Successfully














