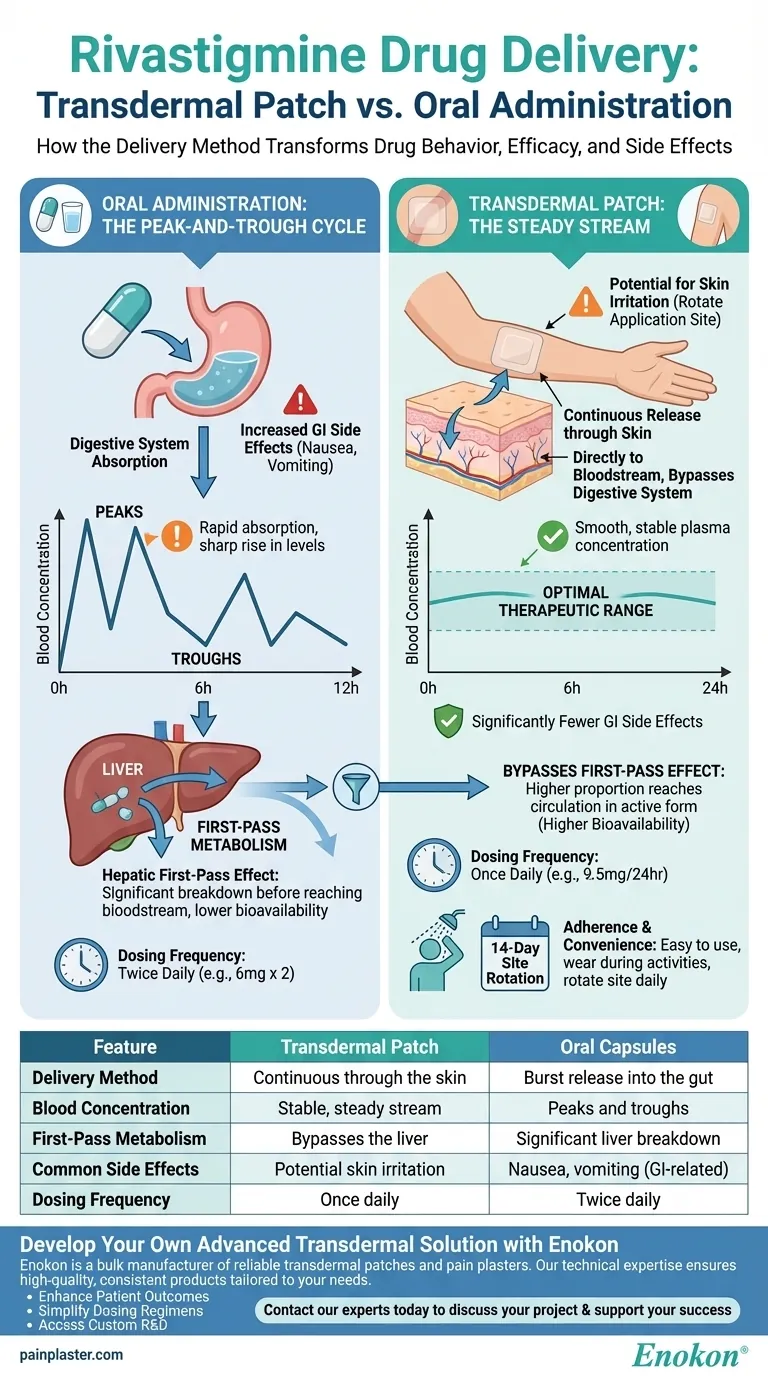
The fundamental difference between the rivastigmine patch and oral capsules is the delivery method, which dramatically changes how the drug behaves in your body. The transdermal patch delivers the medication continuously and steadily through the skin, while oral capsules release it all at once into the digestive system, causing drug levels to rise and fall sharply.
The rivastigmine transdermal patch is engineered to solve the core problem of oral administration: it provides the same therapeutic effect in the brain as the highest oral dose but with significantly fewer gastrointestinal side effects by maintaining a smooth, stable drug concentration in the bloodstream.
The Journey of the Medication: Skin vs. Stomach
To understand the practical differences, it's helpful to visualize the two distinct paths the medication can take to enter your system.
Oral Administration: The Peak-and-Trough Cycle
When you swallow a rivastigmine capsule, it travels through your gastrointestinal (GI) tract. The drug is absorbed relatively quickly, causing a sharp peak in its concentration in your blood.
As your body metabolizes the drug over the next several hours, the concentration declines, leading to a trough, or low point, before the next dose. This cycle of peaks and troughs is a standard feature of oral medication.
Transdermal Patch: The Steady Stream
The transdermal patch works like a drip irrigation system. It adheres to your skin and releases a controlled, continuous amount of rivastigmine over a full 24-hour period.
This medication is absorbed through the skin directly into the bloodstream, creating a smooth and stable plasma concentration. It completely avoids the dramatic peaks and troughs associated with oral doses.
Bypassing the "First Pass": A Key Advantage
The delivery route doesn't just affect the stability of the drug levels; it also affects how much of the drug is available for your body to use.
What is First-Pass Metabolism?
When a drug is taken orally, it is absorbed from the gut and travels first to the liver. The liver acts as a filter, breaking down a significant portion of the medication before it ever reaches the rest of the bloodstream. This is known as the hepatic first-pass effect.
The Patch's Direct Route
By delivering rivastigmine through the skin, the transdermal patch bypasses the digestive system and the liver's first-pass effect.
This means a higher proportion of the drug—what's known as higher bioavailability—reaches your systemic circulation in its active form.
The Impact on Dosing and Efficacy
This efficiency is why the doses for the patch and capsules are not directly equivalent. The 9.5 mg/24-hour patch provides comparable drug exposure to the brain as the maximum recommended oral dose of 12 mg/day (taken as 6 mg twice daily). The patch achieves the same therapeutic goal with a lower total dose.
Understanding the Trade-offs and Practical Realities
Each method has clear benefits and potential downsides that are important to consider.
Reduced Gastrointestinal Side Effects
The primary benefit of the patch's steady, non-GI delivery is a significant reduction in side effects like nausea and vomiting. These issues are often triggered by the high peak concentrations and direct irritation of the digestive tract caused by oral medication.
The Potential for Skin Irritation
The main downside of the transdermal patch is the potential for local skin reactions at the application site.
To minimize this risk, it is critical to apply the patch to clean, dry, and hairless skin. You must rotate the application site daily and not use the same spot again for at least 14 days.
Adherence and Convenience
For many, applying a single patch once a day is simpler and easier to remember than taking pills multiple times a day, which can improve patient compliance. The patch can also be worn during normal activities like showering, bathing, or swimming.
Making the Right Choice for Your Goal
The decision between the patch and oral capsules depends on balancing efficacy, side effects, and personal preference.
- If your primary focus is minimizing gastrointestinal side effects: The transdermal patch is explicitly designed to reduce issues like nausea by providing steady drug delivery that bypasses the digestive system.
- If your primary focus is ensuring treatment efficacy with maximum convenience: The patch offers comparable brain exposure to the highest oral dose but only requires a single, once-daily application.
- If you have very sensitive skin or have experienced irritation from adhesives: Oral administration remains a viable alternative, though it may require more careful management to mitigate potential GI side effects.
Understanding how each method works empowers you to have a more informed discussion with your healthcare provider about the best treatment path for your specific situation.
Summary Table:
| Feature | Transdermal Patch | Oral Capsules |
|---|---|---|
| Delivery Method | Continuous through the skin | Burst release into the gut |
| Blood Concentration | Stable, steady stream | Peaks and troughs |
| First-Pass Metabolism | Bypasses the liver | Significant liver breakdown |
| Common Side Effects | Potential skin irritation | Nausea, vomiting (GI-related) |
| Dosing Frequency | Once daily | Twice daily |
Develop Your Own Advanced Transdermal Solution with Enokon
Are you a healthcare or pharmaceutical distributor or brand looking to leverage the benefits of transdermal delivery? Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise ensures high-quality, consistent products tailored to your needs.
Partner with us to:
- Enhance Patient Outcomes: Create therapies with improved efficacy and reduced side effects.
- Simplify Dosing Regimens: Develop convenient, once-daily options that boost patient compliance.
- Access Custom R&D: Benefit from our experience in custom formulation and development to bring your unique product vision to life.
Contact our experts today to discuss your transdermal project and discover how we can support your success.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine














