The rotigotine transdermal system is a matrix-type patch that delivers medication directly through the skin over a 24-hour period. The drug is integrated into an adhesive polymer matrix, which allows for a continuous and controlled release. This design enables the medication to bypass the digestive system, where it would otherwise be extensively broken down, and enter the bloodstream directly.
The core advantage of the rotigotine patch is its ability to overcome the body's natural gastrointestinal metabolism. By delivering a steady, controlled dose directly through the skin, it ensures stable and consistent drug levels in the brain to effectively manage symptoms.
How the Patch Delivers Rotigotine
The effectiveness of the rotigotine system lies in the sophisticated design of the transdermal patch and its interaction with the skin. It is a multi-step process designed for stability and consistency.
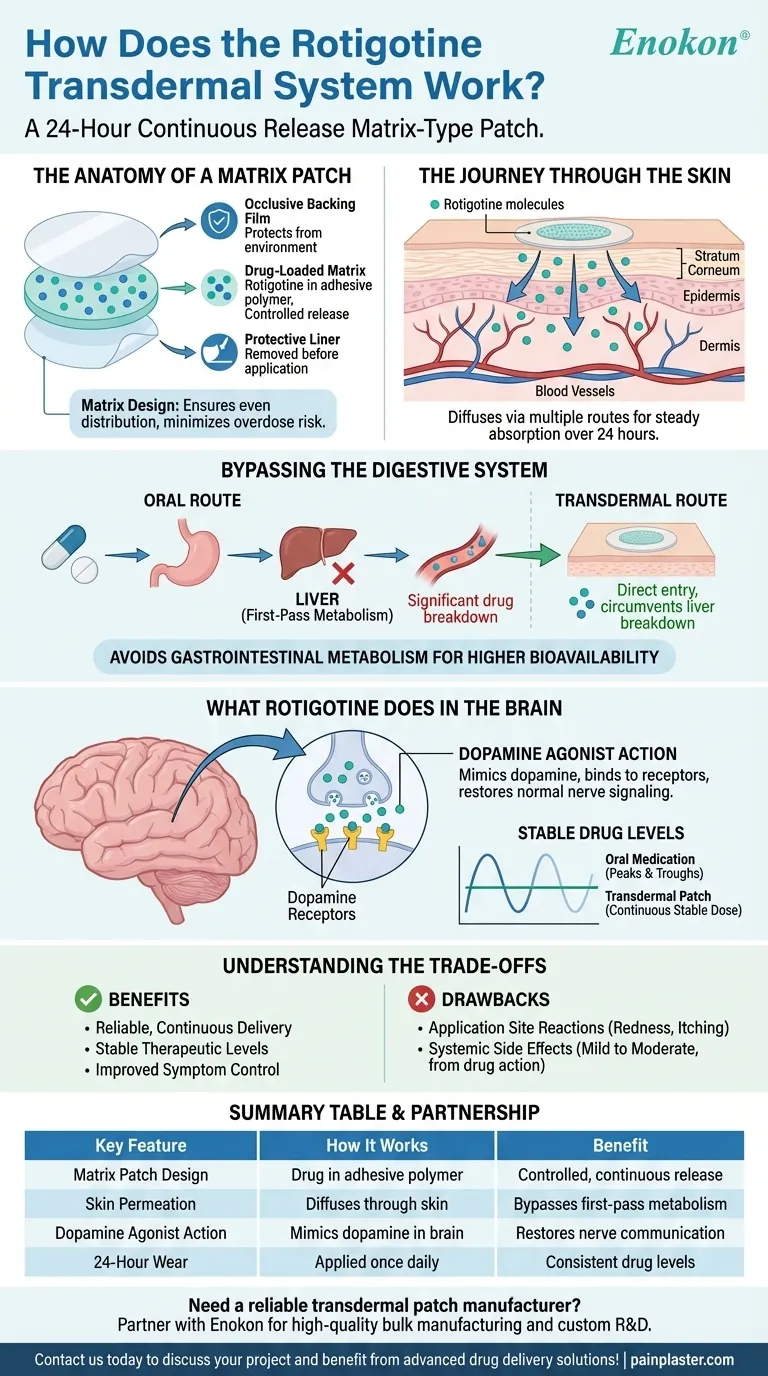
The Anatomy of a Matrix Patch
A matrix-type patch, like the one used for rotigotine, has three primary layers.
- An occlusive backing film protects the patch from the outside environment.
- A drug-loaded matrix contains the active ingredient, rotigotine, evenly distributed within an adhesive polymer.
- A protective liner is peeled away just before application to expose the adhesive matrix.
This matrix design is crucial for safety and efficacy, as the even distribution of the drug minimizes the risk of accidental overdose or "dose dumping."
The Journey Through the Skin
Once the patch is applied, the drug begins its journey into the body. Rotigotine diffuses from the adhesive matrix into the outermost layer of the skin, the stratum corneum.
From there, it permeates deeper through several routes—between cells, through cells, and via hair follicles. This process is controlled by the specific formulation of the matrix, which dictates the rate of release.
Bypassing the Digestive System
The primary reason for using a transdermal patch for rotigotine is to avoid the gastrointestinal tract.
If taken orally, a significant portion of the drug would be destroyed by the liver in a process known as first-pass metabolism. The patch circumvents this entirely, allowing more of the active drug to reach the bloodstream.
Ensuring a Continuous, Stable Dose
The matrix system is engineered to release the drug at a slow, constant rate over 24 hours.
This provides highly stable drug levels in the blood, avoiding the peaks and troughs often associated with oral medications taken at intervals. This consistency is critical for managing chronic conditions.
What Rotigotine Does in the Brain
Once rotigotine enters the bloodstream, it travels to the central nervous system to perform its function. The drug is designed to address a specific chemical imbalance in the brain.
A Dopamine Agonist
Rotigotine is a dopamine agonist. This means it mimics the effects of dopamine, a natural substance in the brain essential for regulating movement, motivation, and mood.
Binding to Receptors
The medication selectively binds to dopamine receptors in the brain. By activating these receptors, it essentially "fills in" for a lack of natural dopamine.
This dopaminergic stimulation helps restore more normal communication between nerve cells, which is the ultimate therapeutic goal.
Understanding the Trade-offs
Like any medical treatment, the rotigotine transdermal system has distinct advantages and potential downsides that are important to understand.
The Benefit: Consistency and Efficacy
The key benefit is the reliable and continuous drug delivery. This avoids the digestive system and provides stable therapeutic levels, which can lead to better symptom control than intermittent oral dosing.
The Drawback: Skin Reactions
The most common side effect is directly related to the delivery method. Application site reactions, such as redness, itching, or mild irritation, can occur because the patch adheres to the skin for 24 hours.
Systemic Side Effects
Other side effects are not from the patch itself but from the drug's dopaminergic action in the brain. These are generally mild to moderate and are a result of the drug doing its intended job of stimulating dopamine receptors throughout the central nervous system.
How to Apply This to Your Understanding
Understanding how the technology works empowers you to better manage your treatment.
- If your primary focus is the delivery method: The key is that the matrix patch provides a steady, 24-hour dose through the skin, completely bypassing the digestive system.
- If your primary focus is the drug's action: Recognize that rotigotine works by acting as a substitute for dopamine in the brain, helping to restore normal nerve cell signaling.
- If your primary concern is side effects: It is useful to distinguish between issues caused by the patch on the skin and those caused by the drug's effects on the brain.
Ultimately, the rotigotine transdermal system is an advanced drug delivery technology designed to provide consistent therapeutic effects by working with your body's natural absorption processes.
Summary Table:
| Key Feature | How It Works | Benefit |
|---|---|---|
| Matrix Patch Design | Drug is evenly distributed in an adhesive polymer layer. | Provides controlled, continuous release; minimizes risk of dose dumping. |
| Skin Permeation | Rotigotine diffuses through the stratum corneum into the bloodstream. | Bypasses first-pass metabolism in the liver, increasing drug availability. |
| Dopamine Agonist Action | Mimics dopamine by binding to receptors in the brain. | Helps restore nerve cell communication for movement and mood regulation. |
| 24-Hour Wear | Patch is applied once daily for consistent drug levels. | Avoids peaks and troughs associated with oral medications. |
Need a reliable transdermal patch manufacturer? Partner with Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise ensures custom R&D and development tailored to your needs. Contact us today to discuss your project and benefit from our advanced drug delivery solutions!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














