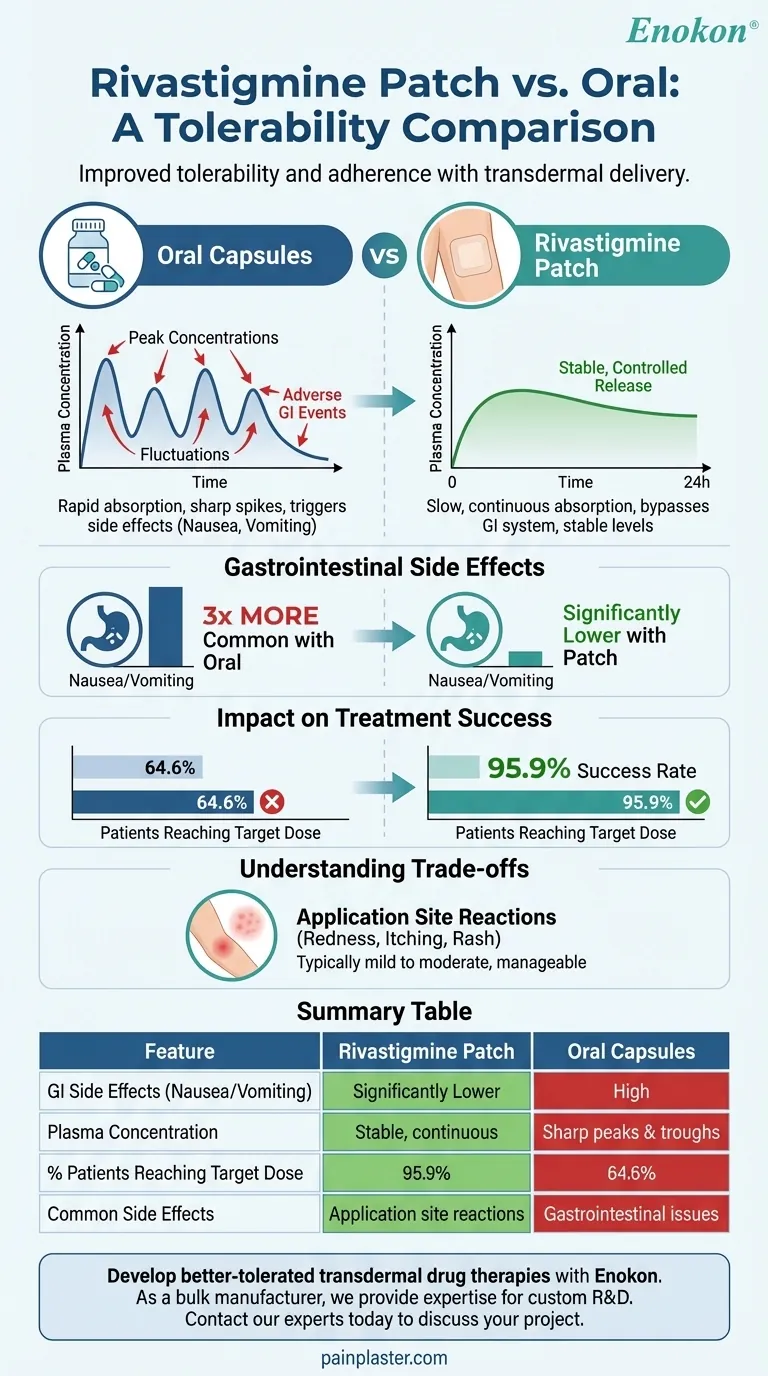
In short, the rivastigmine patch is significantly better tolerated than the oral capsule formulation. This improved tolerability is most pronounced in the reduction of gastrointestinal side effects, such as nausea and vomiting, which are common with the oral version.
The core difference lies in the drug delivery method. The transdermal patch provides slow, continuous absorption through the skin, avoiding the sharp peaks in plasma concentration that occur with oral dosing and trigger adverse gastrointestinal events.
Why the Patch Offers Better Tolerability
The improved side effect profile of the rivastigmine patch is not accidental; it is a direct result of its transdermal delivery mechanism. This method fundamentally changes how the body processes the medication compared to oral capsules.
Stable Plasma Concentrations
With oral administration, the drug is absorbed rapidly, leading to a sharp spike in its concentration in the bloodstream. This peak is often associated with adverse effects.
The patch, in contrast, releases rivastigmine slowly and continuously over 24 hours. This creates much smoother and more stable plasma levels, minimizing the fluctuations that can cause side effects.
Bypassing the Gastrointestinal System
By delivering the drug directly through the skin into the bloodstream, the patch bypasses the digestive system. This avoids the high initial concentration of the drug in the stomach and intestines.
This is critical because cholinergic gastrointestinal events are the most common dose-limiting side effects of oral rivastigmine.
A Measured Reduction in Nausea and Vomiting
The clinical data is clear. Studies show that the 9.5 mg/24-hour patch results in three times fewer reports of nausea and vomiting compared to the equivalent 12 mg/day oral capsule dose.
The Practical Impact on Treatment Success
Better tolerability is not just about patient comfort; it directly impacts the effectiveness of the treatment by improving adherence and ensuring patients can receive an optimal therapeutic dose.
Higher Achievement of Target Doses
Because the side effects are less severe, patients using the patch are far more likely to reach and maintain their target therapeutic dose.
One study found that 95.9% of patients using the patch successfully reached the target dose, compared to only 64.6% of those taking oral capsules.
Comparable Efficacy with Fewer Downsides
Importantly, this improved tolerability does not compromise the drug's efficacy. The 9.5 mg/24-hour patch provides a comparable level of drug exposure to the 12 mg/day oral dose, ensuring therapeutic goals can be met.
Understanding the Trade-offs
While the patch is generally better tolerated, no delivery method is entirely without potential side effects. The focus of adverse events simply shifts from gastrointestinal to dermatological.
Application Site Reactions
The most common adverse events associated with the rivastigmine patch are application site reactions, such as redness, itching, or rash.
These reactions are typically mild to moderate in severity and can often be managed by rotating the application site daily.
Overall Adverse Event Profile
Across the board, most adverse events reported with the patch are classified as mild or moderate. The significant reduction in severe GI issues makes it the preferred option for many patients who struggle with the oral form.
Making the Right Choice for Your Goal
The choice between the patch and oral capsules should be guided by the primary goal for the patient's treatment plan.
- If your primary focus is minimizing nausea and vomiting: The patch is the clear superior choice due to its dramatically lower incidence of these specific side effects.
- If your primary focus is treatment adherence and reaching the target dose: The patch offers a significant advantage, as its tolerability profile allows a much higher percentage of patients to stay on their prescribed therapy.
- If your primary focus is managing a patient with sensitive skin: Monitor the application site closely, but for most, the benefits of avoiding severe GI distress will outweigh the risk of mild skin irritation.
Ultimately, the rivastigmine patch offers a more stable and tolerable delivery system that helps more patients achieve their therapeutic goals.
Summary Table:
| Feature | Rivastigmine Patch | Oral Capsules |
|---|---|---|
| GI Side Effects (Nausea/Vomiting) | Significantly Lower | High |
| Plasma Concentration | Stable, continuous | Sharp peaks & troughs |
| % Patients Reaching Target Dose | 95.9% | 64.6% |
| Common Side Effects | Application site reactions | Gastrointestinal issues |
Develop better-tolerated transdermal drug therapies with Enokon. As a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Let us help you create a product that improves patient adherence and outcomes. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
















