The Diclofenac Transdermal Patch is a specialized drug delivery system designed to provide controlled, sustained release of diclofenac through the skin for pain relief. Each 50 sq. cm patch contains 100 mg of Diclofenac Diethylamine embedded in a polymer matrix that regulates drug release over 24 hours. By blocking prostaglandin production, it reduces inflammation and pain at the application site while minimizing systemic side effects. Key components include an impermeable backing layer, drug reservoir, rate-controlling membrane, and skin-contact adhesive. Proper application involves clean, dry skin placement away from sensitive areas, with careful handling and disposal to ensure safety and efficacy.
Key Points Explained:
-
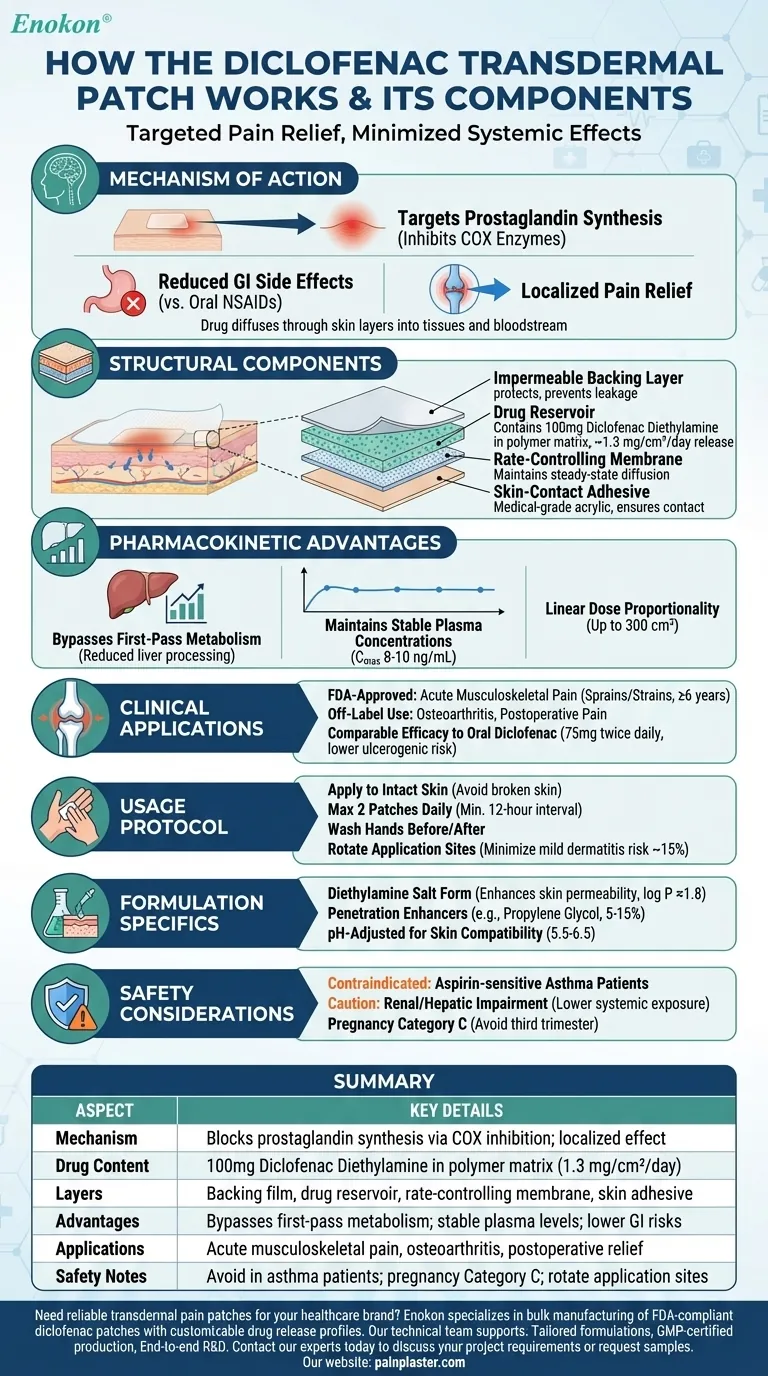
Mechanism of Action
- Targets prostaglandin synthesis by inhibiting cyclooxygenase (COX) enzymes
- Provides localized pain relief while reducing gastrointestinal side effects compared to oral NSAIDs
- Drug molecules diffuse through skin layers into underlying tissues and bloodstream
-
Structural Components
- Backing layer: Polyester or polyethylene film that prevents drug leakage and protects from environmental factors
- Drug reservoir: Contains 100 mg Diclofenac Diethylamine suspended in polymer matrix (typically acrylic or silicone-based)
- Release-controlling membrane: Polymeric film that maintains steady-state drug diffusion (≈1.3 mg/cm²/day)
- Adhesive layer: Medical-grade acrylic adhesive ensuring skin contact while contributing to controlled release
-
Pharmacokinetic Advantages
- Bypasses first-pass metabolism, reducing liver processing
- Maintains stable plasma concentrations (Cmax 8-10 ng/mL)
- Shows linear dose proportionality up to 300 cm² application area
-
Clinical Applications
- FDA-approved for acute musculoskeletal pain (sprains/strains) in patients ≥6 years
- Off-label use for osteoarthritis and postoperative pain management
- Provides comparable efficacy to oral diclofenac 75 mg twice daily with lower ulcerogenic risk
-
Usage Protocol
- Apply to intact skin over painful area (avoiding broken skin)
- Maximum 2 patches daily (minimum 12-hour interval)
- Wash hands before/after application to prevent accidental eye exposure
- Rotate application sites to minimize skin irritation (≈15% incidence of mild dermatitis)
-
Formulation Specifics
- Diethylamine salt form enhances skin permeability (log P ≈1.8)
- Contains penetration enhancers like propylene glycol (5-15% concentration)
- pH-adjusted to 5.5-6.5 for skin compatibility
-
Safety Considerations
- Contraindicated in aspirin-sensitive asthma patients
- Requires caution in renal/hepatic impairment (lower systemic exposure than oral forms)
- Pregnancy Category C (avoid third trimester due to fetal cardiovascular risks)
Summary Table:
| Aspect | Key Details |
|---|---|
| Mechanism | Blocks prostaglandin synthesis via COX inhibition; localized effect |
| Drug Content | 100 mg Diclofenac Diethylamine in polymer matrix (1.3 mg/cm²/day release) |
| Layers | Backing film, drug reservoir, rate-controlling membrane, skin adhesive |
| Advantages | Bypasses first-pass metabolism; stable plasma levels; lower GI risks |
| Applications | Acute musculoskeletal pain, osteoarthritis (off-label), postoperative relief |
| Safety Notes | Avoid in asthma patients; pregnancy Category C; rotate application sites |
Need reliable transdermal pain patches for your healthcare brand?
Enokon specializes in bulk manufacturing of FDA-compliant diclofenac patches with customizable drug release profiles. Our technical team supports:
- Tailored formulations for specific pain management needs
- GMP-certified production for consistent quality
- End-to-end R&D for novel transdermal solutions
Contact our experts today to discuss your project requirements or request samples.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














