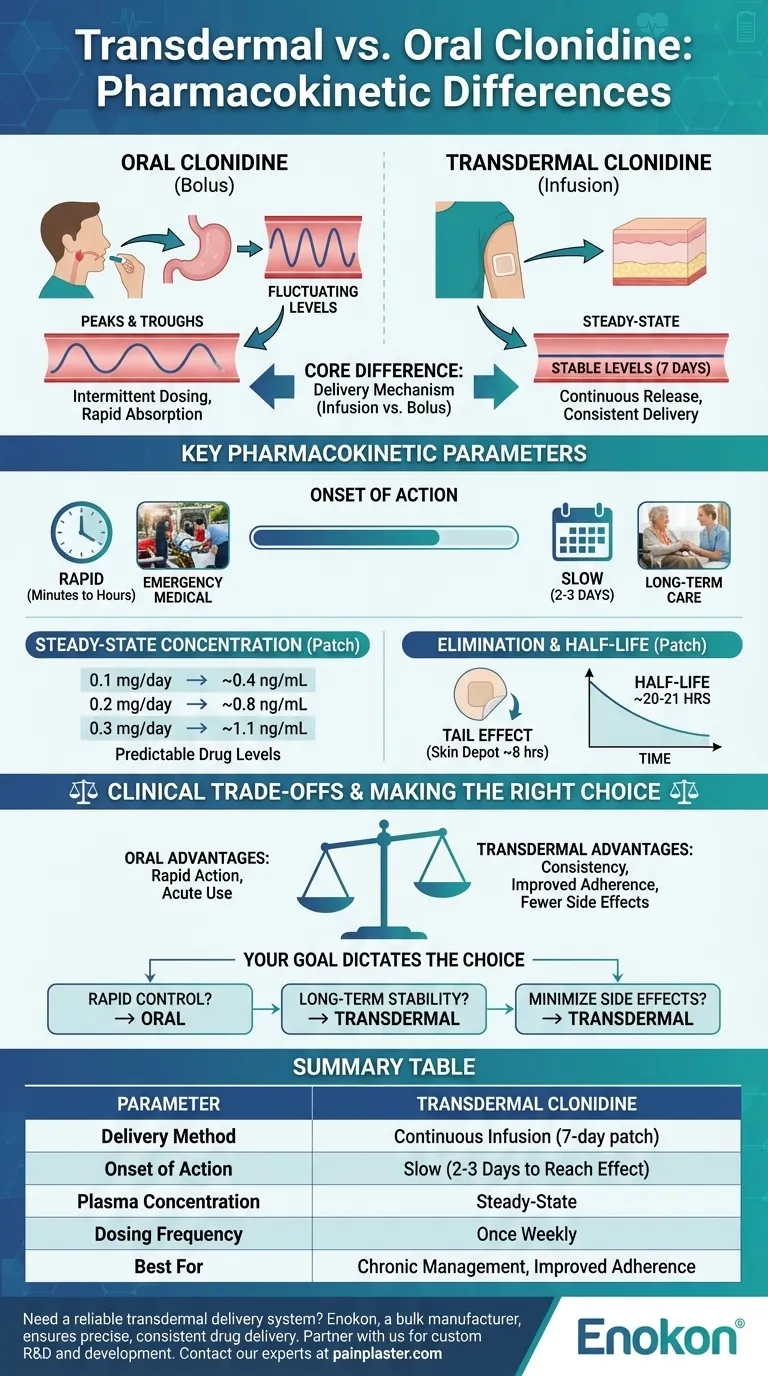
In essence, the primary pharmacokinetic difference lies in the delivery mechanism: oral clonidine provides intermittent, fluctuating drug levels, whereas transdermal clonidine delivers a continuous, stable amount of the drug over a seven-day period. This fundamental distinction impacts everything from the onset of action to the consistency of the therapeutic effect.
The choice between transdermal and oral clonidine represents a classic clinical trade-off. You must weigh the rapid action of oral tablets against the consistent, steady-state blood levels offered by the transdermal patch, which can improve adherence and minimize side effects.

The Core Difference: Infusion vs. Bolus Dosing
The method of drug delivery creates two entirely different pharmacokinetic profiles. Oral tablets act as a "bolus," while the patch acts as a slow "infusion."
Oral Clonidine: The "Peak and Trough" Effect
When a patient takes an oral tablet, the drug is absorbed relatively quickly, causing a sharp rise in blood concentration, known as a peak. As the body metabolizes and eliminates the drug, the concentration falls, creating a trough before the next dose. This cycle of peaks and troughs is inherent to intermittent oral dosing.
Transdermal Clonidine: The "Steady-State" Advantage
The transdermal patch releases clonidine at a constant rate. This process is similar to a continuous intravenous infusion, bypassing the fluctuations seen with oral administration. The result is a stable, steady-state plasma concentration that is maintained throughout the 7-day application period, eliminating the peaks and troughs.
Key Pharmacokinetic Parameters Compared
These two delivery systems have distinct profiles for how quickly they work, how they are absorbed, and how they are eliminated.
Onset of Action
The most significant difference is the time it takes to achieve a therapeutic effect. Oral clonidine acts quickly, making it suitable for acute situations. In contrast, the transdermal patch has a notable delay, requiring two to three days to reach therapeutic blood levels.
Steady-State Concentration
Once achieved, the patch provides predictable drug levels. Mean steady-state concentrations are directly proportional to the patch strength:
- 0.1 mg/day patch: ~0.4 ng/mL
- 0.2 mg/day patch: ~0.8 ng/mL
- 0.3 mg/day patch: ~1.1 ng/mL
Elimination and Half-Life
After removing the patch, the clonidine already absorbed into the layers of the skin continues to be released into the bloodstream. This creates a "tail" effect where plasma levels remain constant for about eight hours before starting to decline. The drug then has a long elimination half-life of approximately 20 to 21 hours.
Understanding the Clinical Trade-offs
The pharmacokinetic differences create clear advantages and disadvantages for each formulation, guiding the clinical decision-making process.
Why Choose the Patch? Consistency and Adherence
The primary benefit of the transdermal system is consistency. Stable blood levels provide smoother blood pressure control and may reduce side effects, such as sedation, which are often associated with the peak concentrations of oral doses. Furthermore, a once-weekly application dramatically improves patient adherence compared to multiple daily pills.
The Limitation: Slow Onset
The 2-3 day delay to reach effectiveness makes the patch entirely unsuitable for hypertensive emergencies or any situation requiring rapid dose titration. It is designed exclusively for chronic, long-term management.
The Challenge: Discontinuation
The slow elimination after patch removal means the drug's effects do not stop immediately. This must be considered when discontinuing the medication or switching to another therapy, as the effects will linger for several days.
Making the Right Choice for Your Goal
Your clinical objective should dictate which formulation is appropriate.
- If your primary focus is rapid blood pressure control: Oral clonidine is the only suitable choice due to its fast onset of action.
- If your primary focus is long-term, stable management and patient adherence: The transdermal patch is superior, providing consistent drug levels with a convenient once-weekly application.
- If your primary focus is minimizing side effects from fluctuating drug levels: The transdermal patch's steady-state delivery avoids the concentration peaks that can trigger adverse effects.
Understanding these distinct pharmacokinetic profiles empowers you to select the delivery system that best aligns with the specific therapeutic objective.
Summary Table:
| Parameter | Oral Clonidine | Transdermal Clonidine |
|---|---|---|
| Delivery Method | Bolus (Intermittent) | Continuous Infusion (7-day patch) |
| Onset of Action | Rapid (Minutes to Hours) | Slow (2-3 Days to Reach Effect) |
| Plasma Concentration | Peaks and Troughs | Steady-State (e.g., 0.3 mg/day patch ≈ 1.1 ng/mL) |
| Dosing Frequency | Multiple Times Daily | Once Weekly |
| Best For | Acute Situations, Rapid Titration | Chronic Management, Improved Adherence |
Need a reliable transdermal delivery system for your pharmaceutical product?
As Enokon, a bulk manufacturer of high-quality transdermal patches, we understand the critical importance of precise, consistent drug delivery. Our technical expertise in custom R&D and development ensures your transdermal product—like a clonidine patch—achieves the desired steady-state pharmacokinetic profile for optimal patient outcomes.
Partner with us to:
- Develop custom transdermal solutions for healthcare and pharma brands.
- Leverage our manufacturing reliability for pain plasters and medicated patches.
- Benefit from our technical support from formulation to production.
Contact our experts today to discuss your transdermal project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints















