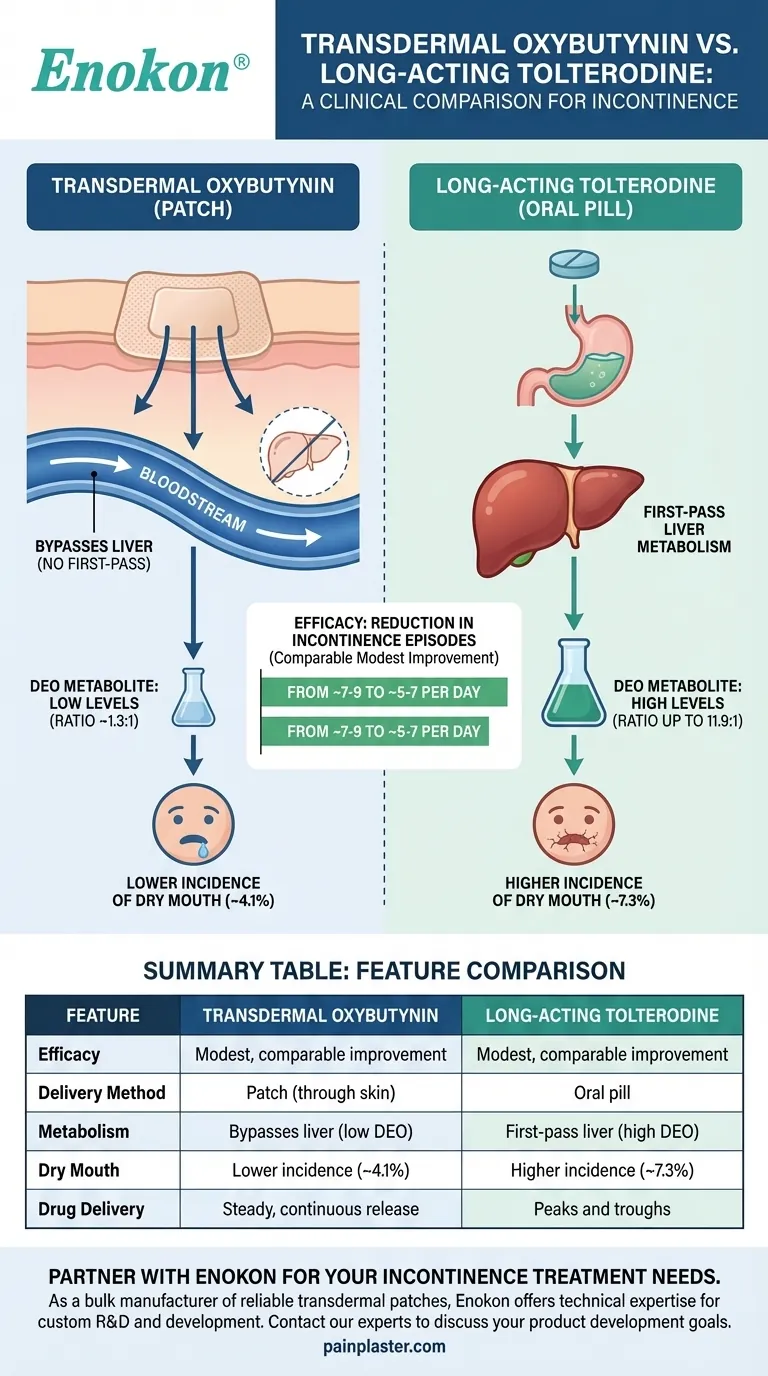
From a clinical standpoint, transdermal oxybutynin and long-acting tolterodine offer comparable effectiveness in reducing incontinence episodes. The fundamental difference between them lies not in their efficacy, but in how the body processes them, which directly impacts their side effect profiles, particularly the incidence of dry mouth.
While both medications provide a similar modest reduction in incontinence, transdermal oxybutynin's key advantage is its delivery method, which bypasses the liver and significantly lowers the concentration of the metabolite responsible for common side effects like dry mouth.

Efficacy: A Tale of Two Equals
When evaluating the primary goal of reducing incontinence, the evidence shows a similar outcome for both treatments.
Similar Reduction in Episodes
Both transdermal oxybutynin and long-acting tolterodine have been shown to produce a modest but statistically significant decrease in daily incontinence events.
Studies show they can reduce the number of episodes from a baseline of 7 to 9 per day down to a range of 5 to 7 per day. This improvement is notably better than a placebo.
The Critical Difference: Metabolism and Side Effects
The real distinction emerges when you look at the drug's journey through the body. The delivery mechanism—a patch versus a pill—is the source of their different side effect profiles.
Bypassing First-Pass Metabolism
Oral medications like tolterodine are absorbed through the digestive system and must first pass through the liver before entering the bloodstream. This is known as first-pass metabolism.
The transdermal oxybutynin patch delivers the medication directly through the skin into the bloodstream, completely bypassing this initial liver metabolism.
The Role of the DEO Metabolite
When oral oxybutynin is processed by the liver, it creates a large amount of a metabolite called N-desethyloxybutynin (DEO). This substance is strongly associated with the anticholinergic side effects common to this class of drugs, especially dry mouth.
A Stark Contrast in Metabolite Levels
Because the patch bypasses the liver, it produces much lower levels of DEO. The ratio of active drug to the DEO metabolite for transdermal oxybutynin is approximately 1.3-to-1.
For oral forms, this ratio can be as high as 11.9-to-1, meaning there is a much higher concentration of the side-effect-causing metabolite in the system.
Lower Incidence of Dry Mouth
This dramatic difference in metabolism often translates to a better patient experience. One study found the rate of dry mouth was nearly half with the patch (4.1%) compared to extended-release oral tolterodine (7.3%).
Understanding the Trade-offs
Choosing between these medications requires balancing clinical benefits with practical considerations like cost and delivery preference.
Consistent Drug Delivery
The transdermal patch provides a continuous and steady delivery of medication. This minimizes the peaks and troughs in plasma levels that can occur with oral dosing, potentially leading to more stable symptom control.
Cost Considerations
The cost of transdermal oxybutynin is generally similar to that of brand-name, extended-release oral medications like tolterodine.
However, both are significantly more expensive than the generic, immediate-release version of oral oxybutynin, which can cost as little as $15 to $30 per month.
Making the Right Choice for Your Goal
Your decision should be guided by the patient's specific priorities and tolerance for potential side effects.
- If your primary focus is efficacy: Both transdermal oxybutynin and long-acting tolterodine provide a similar, modest benefit in reducing incontinence episodes.
- If your primary focus is minimizing dry mouth: Transdermal oxybutynin holds a distinct pharmacological advantage and is often the preferred choice for patients sensitive to this side effect.
- If your primary focus is managing cost: While these two drugs are similarly priced, generic oral alternatives are a much more economical option if their side effect profile is tolerable.
Ultimately, understanding the metabolic pathway is key to selecting the treatment that best balances efficacy with patient quality of life.
Summary Table:
| Feature | Transdermal Oxybutynin | Long-Acting Tolterodine |
|---|---|---|
| Efficacy (Reduction in episodes) | Modest, comparable improvement | Modest, comparable improvement |
| Delivery Method | Patch (through skin) | Oral pill |
| Metabolism | Bypasses liver (low DEO metabolite) | First-pass liver metabolism (high DEO metabolite) |
| Common Side Effect: Dry Mouth | Lower incidence (~4.1%) | Higher incidence (~7.3%) |
| Drug Delivery | Steady, continuous release | Peaks and troughs with dosing |
Partner with Enokon for Your Incontinence Treatment Needs
As a bulk manufacturer of reliable transdermal patches for healthcare and pharmaceutical distributors, Enokon can help you bring advanced treatment options like transdermal oxybutynin to market. Benefit from our technical expertise for custom R&D and development of pain plasters and medicated patches.
Contact our experts today to discuss how we can support your product development goals.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the limitations of transdermal patches for period problems? Key Delivery Challenges Explained
- How does the controlled release mechanism work in pain patches? Precision Drug Delivery Explained
- What is the purpose of PEG as a plasticizer in transdermal patches? Enhance Patch Flexibility & Durability
- What is serotonin syndrome, and how is it related to selegiline transdermal patch? Understanding the Critical Risks
- What key performance criteria must the pressure-sensitive adhesive layer meet in a transdermal drug delivery system?
- What are Chinese herbal medicated pain patches? Natural Relief for Muscle & Joint Pain
- Why is the daily rotation of application sites required when using Asenapine Transdermal Patches? Protect Skin Health
- What is the function of Polyvinyl Alcohol (PVA) aqueous solution in the production of Ketotifen transdermal patches? Explained














