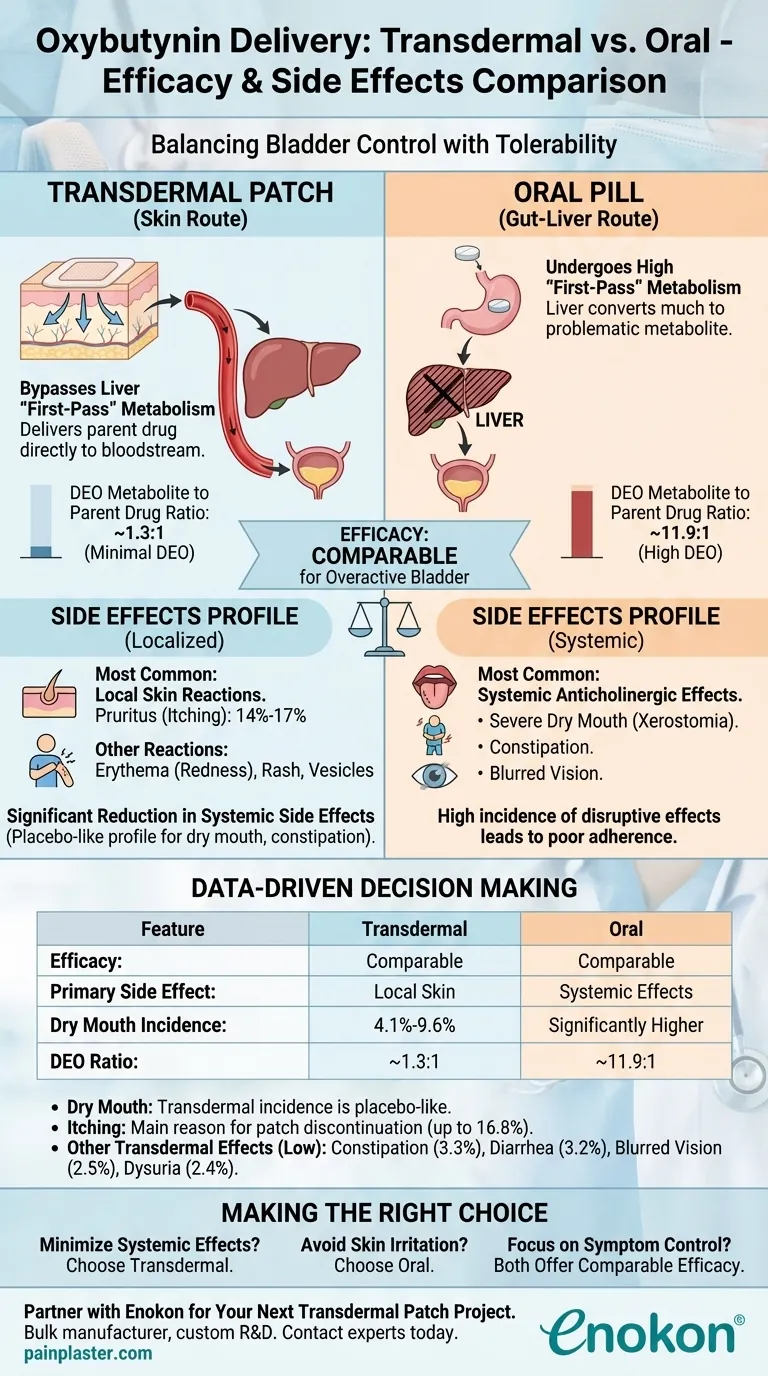
In short, transdermal oxybutynin offers comparable efficacy to oral formulations for managing overactive bladder, but with a significantly different side effect profile. It effectively trades the well-known systemic side effects of oral medications, like severe dry mouth, for localized skin reactions at the application site.
The core difference comes down to metabolism. By absorbing through the skin, transdermal oxybutynin bypasses the liver's "first-pass" effect, drastically reducing the metabolite that causes most systemic side effects, while maintaining its therapeutic effectiveness on the bladder.
Why Delivery Method Dictates the Patient Experience
To understand the choice between oral and transdermal oxybutynin, you must look beyond the active ingredient and focus on how it enters the body. This single factor changes everything.
The First-Pass Metabolism Problem
When you swallow an oxybutynin pill, it is absorbed through the gut and passes directly through the liver before entering general circulation. This is called first-pass metabolism.
The liver is highly efficient at breaking down oxybutynin into a metabolite known as N-desethyloxybutynin (DEO).
The Role of the DEO Metabolite
This DEO metabolite is the primary culprit behind the classic anticholinergic side effects associated with oral oxybutynin: severe dry mouth, constipation, and blurred vision.
For oral formulations, the ratio of this problematic metabolite to the parent drug in the blood can be as high as 11.9 to 1.
How Transdermal Bypasses the Issue
The transdermal patch delivers oxybutynin directly through the skin into the bloodstream. This route completely bypasses the liver's first-pass effect.
As a result, far less of the drug is converted into DEO. The ratio of metabolite to parent drug for the transdermal form is only about 1.3 to 1.
Consistent Delivery, Fewer Fluctuations
The patch also provides slow, continuous drug delivery. This avoids the peaks and troughs in plasma concentration that occur with oral dosing, leading to a more stable and better-tolerated therapeutic effect.
Understanding the Trade-offs: Systemic vs. Local Side Effects
The choice between oral and transdermal is a clear trade-off. You are selecting which category of side effects is more acceptable.
Systemic Anticholinergic Effects (The Oral Problem)
Because transdermal delivery produces so much less DEO, its profile for systemic side effects is comparable to a placebo. This means a dramatic reduction in:
- Dry mouth (Xerostomia)
- Constipation
- Blurred vision
For patients who abandon oral therapy due to these disruptive effects, the transdermal patch offers a significant advantage.
Localized Application-Site Reactions (The Transdermal Problem)
The primary drawback of the transdermal patch is its effect on the skin. The most common side effects are mild to moderate reactions at the application site.
The most frequently reported issue is pruritus (itching), affecting roughly 14% to 17% of users.
Other potential skin reactions include erythema (redness) and, less commonly, a rash or vesicles.
A Closer Look at the Data
While both forms are effective, the reported side effects paint a clear picture of their differences.
Dry Mouth (Xerostomia)
For the transdermal patch, the incidence of dry mouth is between 4.1% and 9.6%, a rate considered similar to placebo. This is the most significant advantage over oral therapy.
Application-Site Itching
Itching is the main reason for discontinuing the transdermal patch. With an incidence rate of up to 16.8%, it is a key factor to consider for any patient.
Other Potential Side Effects
Other reported side effects for transdermal oxybutynin are relatively low, including constipation (3.3%), diarrhea (3.2%), blurred vision (2.5%), and dysuria (2.4%).
Making the Right Choice for Your Goal
The evidence shows that the decision between oral and transdermal oxybutynin should be based entirely on the individual patient's priorities and tolerance for specific side effects.
- If your primary focus is minimizing systemic side effects: Transdermal oxybutynin is the superior choice, particularly for patients intolerant of the dry mouth and constipation from oral agents.
- If your primary focus is avoiding skin irritation: Oral formulations are the more reliable option, especially for patients with sensitive skin or a history of contact dermatitis.
- If your primary focus is purely on bladder symptom control: Both transdermal and oral formulations provide comparable efficacy.
Ultimately, selecting the right formulation is about aligning the delivery mechanism with the patient's individual profile to achieve the best possible balance of efficacy and tolerability.
Summary Table:
| Feature | Transdermal Oxybutynin | Oral Oxybutynin |
|---|---|---|
| Efficacy | Comparable to oral | Comparable to transdermal |
| Primary Side Effect | Local skin reactions (itching, redness) | Systemic effects (dry mouth, constipation) |
| Dry Mouth Incidence | 4.1% - 9.6% (placebo-like) | Significantly higher |
| Metabolism | Bypasses liver (first-pass) | High first-pass metabolism |
| DEO Metabolite Ratio | ~1.3:1 | ~11.9:1 |
Partner with Enokon for Your Next Transdermal Patch Project
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with the technical expertise needed for custom R&D and development. Benefit from our experience to create effective, patient-friendly transdermal solutions.
Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What foods and substances should be avoided while using selegiline transdermal? A Safety Guide by Dose
- How does the rivastigmine patch compare to oral capsules? Better Tolerability & Easier Dosing
- What are common skin reactions to HRT patches? Managing Irritation & Discomfort
- How does Crosshatched Plate Geometry benefit SEBS transdermal patch testing? Ensure Accurate Rheological Data
- What are the advantages of transdermal drug delivery? Safe, Steady & Non-Invasive Medication
- What physical environment does the Fürst penetration test apparatus provide? Master Depth Profiling in Transdermal R&D
- How does the selegiline transdermal system avoid dietary restrictions? Bypass Gut Issues for Safer Treatment
- What is the potential of a transdermal Alzheimer's vaccine? Exploring a Non-Invasive Breakthrough















