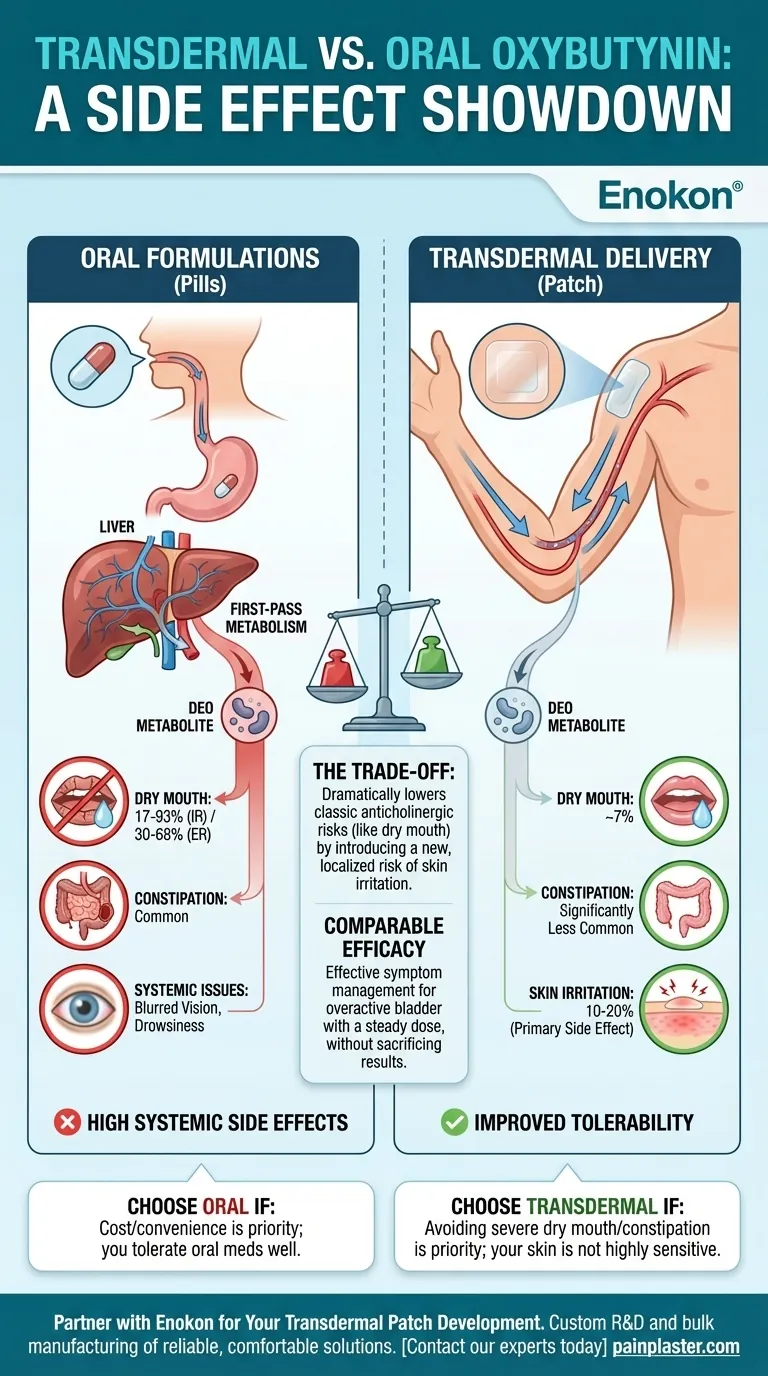
The fundamental difference is that transdermal oxybutynin (a patch or gel) significantly reduces systemic side effects like dry mouth and constipation compared to oral formulations (pills). By delivering the medication directly through the skin, it bypasses the metabolic process in the liver that creates the compounds largely responsible for these adverse effects.
The choice between transdermal and oral oxybutynin is a direct trade-off: The patch dramatically lowers the risk of classic anticholinergic side effects like dry mouth at the cost of introducing a new, localized risk of skin irritation at the application site.
Why Transdermal Delivery Changes the Equation
To understand the difference in side effects, you have to understand the different journeys the medication takes through the body. The route of administration is the most important factor.
Bypassing First-Pass Metabolism
When you swallow an oxybutynin pill, it is absorbed through your gut and passes through the liver before it ever reaches the rest of your bloodstream. This is known as first-pass metabolism.
During this process, the liver metabolizes a large portion of the drug into a different active compound.
The Role of the DEO Metabolite
This new compound is a metabolite called N-desethyloxybutynin (DEO). Unfortunately, DEO is the primary culprit behind the most troublesome anticholinergic side effects, including severe dry mouth, constipation, and cognitive fog.
It has a much higher ratio of side effects to therapeutic benefit than the parent drug, oxybutynin.
A More Direct Path to the Target
Transdermal delivery sends oxybutynin directly into the small blood vessels beneath the skin, allowing it to circulate throughout the body.
This more direct path largely avoids first-pass metabolism in the liver. As a result, far less of the problematic DEO metabolite is produced, leading to a much more tolerable experience for the user.
Side Effects: A Head-to-Head Comparison
The data clearly shows a dramatic difference in the incidence of systemic side effects, while also highlighting the unique downside of the transdermal route.
Dry Mouth: A Drastic Reduction
Dry mouth is the most common reason people stop taking oral oxybutynin. Transdermal delivery makes this side effect significantly less likely.
- Oral Immediate-Release: 17% to 93% of users
- Oral Extended-Release: 30% to 68% of users
- Transdermal Patch: Approximately 7% of users
The side effect profile for the transdermal patch is often comparable to that of a placebo for these systemic issues.
Constipation and Other Systemic Issues
The same metabolic advantage applies to other anticholinergic side effects. Constipation, blurred vision, and drowsiness are all significantly less common with transdermal administration.
Application-Site Reactions: The Transdermal Trade-off
The primary drawback of the patch is localized skin irritation. This is the one area where the transdermal route introduces a new side effect not present with oral forms.
About 10% to 20% of patients using the patch experience application-site reactions like itching or redness. For about one in ten users, this irritation can be significant enough to discontinue use.
Understanding the Trade-offs
Choosing a formulation is not about which one is "better" overall, but which one presents the most acceptable balance of efficacy and side effects for the individual.
Efficacy Remains Comparable
It's critical to know that the improved tolerability of the patch does not come at the cost of effectiveness.
Transdermal oxybutynin demonstrates efficacy comparable to oral agents in managing symptoms of overactive bladder. The delivery system is designed to provide a steady, effective dose of the drug over time.
The Core Decision Point
The decision hinges on what you are more willing to tolerate. Are you trying to escape the debilitating dry mouth from the pills, or do you have highly sensitive skin that might react to an adhesive patch?
This balance between avoiding systemic side effects and risking localized ones is the central trade-off.
Making the Right Choice for Your Situation
By understanding how each formulation works, you can make a more informed decision with your healthcare provider based on your personal health profile and priorities.
- If your primary focus is avoiding systemic side effects like severe dry mouth, constipation, or drowsiness, transdermal oxybutynin is the clearly superior choice.
- If you have very sensitive skin or a known history of adverse reactions to medical adhesives, the risk of application-site irritation is a critical factor to consider.
- If your priority is cost or convenience and you have previously tolerated oral medications well, an oral formulation remains a completely valid and effective option.
Understanding this difference in drug metabolism empowers you to have a more productive conversation with your doctor about the best treatment path for your specific needs.
Summary Table:
| Side Effect | Oral Oxybutynin (Incidence) | Transdermal Oxybutynin (Incidence) |
|---|---|---|
| Dry Mouth | 17-93% (Immediate-Release) 30-68% (Extended-Release) |
~7% |
| Constipation | Common | Significantly Less Common |
| Skin Irritation | Not Applicable | 10-20% (Primary Side Effect) |
| Overall Tolerability | High Systemic Side Effects | Much Improved, Comparable to Placebo for Systemic Issues |
Partner with Enokon for Your Transdermal Patch Development
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon specializes in creating formulations that maximize therapeutic benefits while minimizing side effects, just like the oxybutynin patch discussed. Our technical expertise in custom R&D ensures your healthcare or pharma brand can deliver superior patient experiences through innovative transdermal technology.
Let us help you develop a comfortable, effective transdermal solution for your patients. Contact our experts today to discuss your custom patch needs!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the key clinical benefits of the oxybutynin transdermal system? Superior Tolerability & Steady Relief
- Why might oral and transdermal hormone therapies have different effects on gallbladder disease? Bypass the Liver's First-Pass
- How should transdermal nitroglycerin ointment be stored? Ensure Potency and Safety with Correct Storage
- What were the findings of the pharmacokinetic study comparing rivastigmine patch and capsule? A Smoother, More Tolerable Profile
- Can the scopolamine patch be used during pregnancy? Risks & Safety Guidelines
- How should scopolamine transdermal be stored? Essential Guidelines for Patch Stability
- What are the benefits of using the birth control patch? Discover Weekly Convenience & Health Perks
- What are the potential side effects of using nicotine patches? A Guide to Safe and Effective Use
















