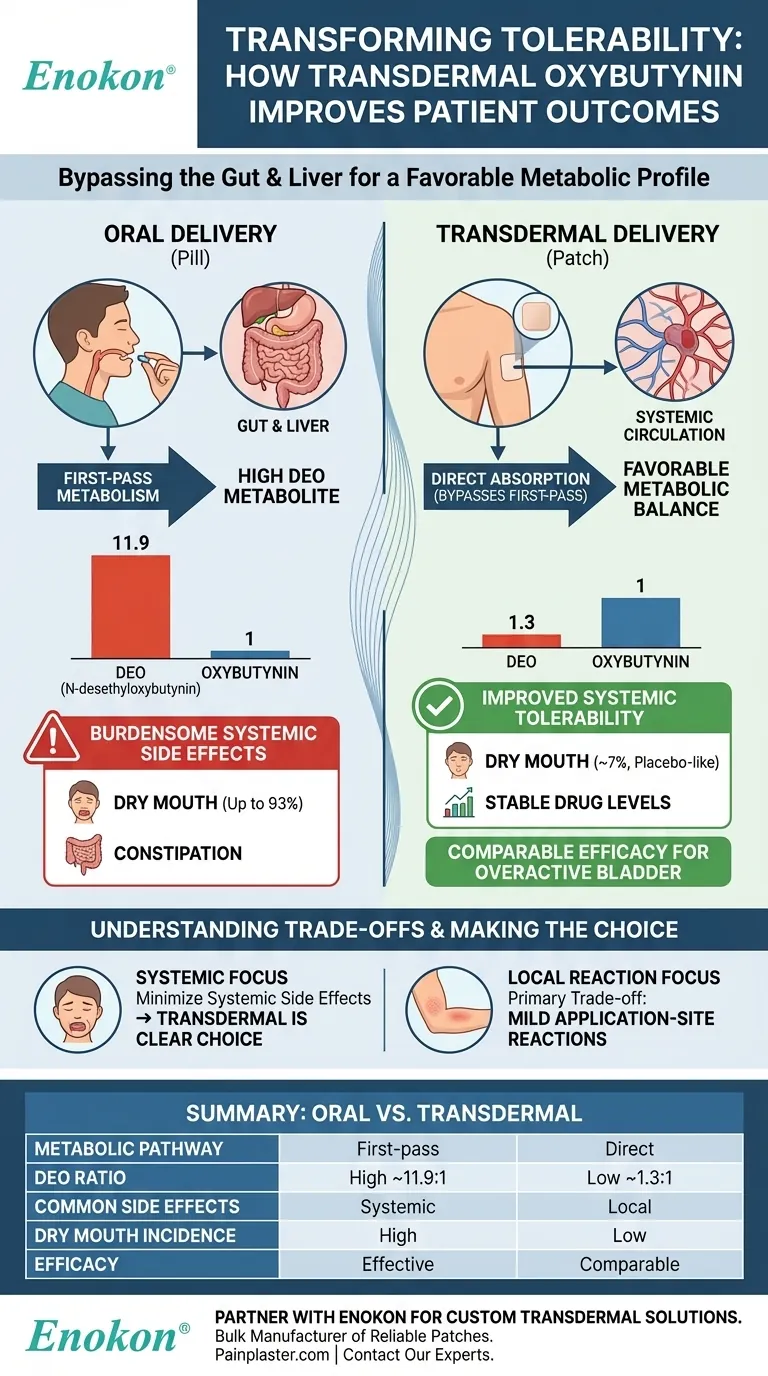
To put it simply, transdermal oxybutynin improves tolerability by delivering the drug directly through the skin, which bypasses the gut and liver. This route dramatically reduces the formation of a metabolite named N-desethyloxybutynin (DEO), which is the primary cause of burdensome side effects like dry mouth and constipation associated with oral formulations.
The core reason for better tolerance is not about the drug itself, but the delivery method. Transdermal administration fundamentally changes how oxybutynin is metabolized, creating a much more favorable balance between the therapeutic parent drug and its side-effect-causing metabolite.

The Problem with Oral Oxybutynin: First-Pass Metabolism
To understand the benefit of the transdermal patch, we must first examine the limitations of the oral route. The issue begins with a process known as the "first-pass effect."
What is First-Pass Metabolism?
When you swallow a pill, the drug is absorbed from the gut and travels directly to the liver before it ever reaches the rest of your body.
The gut wall and liver act as initial processing centers, extensively breaking down or metabolizing the drug. This is the first-pass effect.
The Role of the DEO Metabolite
For oral oxybutynin, this metabolic process creates large quantities of a metabolite called N-desethyloxybutynin (DEO).
While oxybutynin itself is effective at treating bladder symptoms, its DEO metabolite is less effective at this and is strongly associated with causing systemic anticholinergic side effects, particularly dry mouth and constipation.
An Unfavorable Ratio
Oral administration results in a plasma ratio of DEO to oxybutynin as high as 11.9 to 1.
This means for every unit of the therapeutic drug in the bloodstream, there are nearly twelve units of the metabolite primarily responsible for side effects. This poor ratio is the root cause of the tolerability issues with oral pills.
How Transdermal Delivery Solves the Problem
The transdermal patch was engineered specifically to overcome the metabolic disadvantages of the oral route.
Bypassing the Gut and Liver
By delivering oxybutynin through the skin, the drug is absorbed directly into the systemic circulation.
This allows it to bypass the initial, intensive metabolism in the gut and liver, avoiding the first-pass effect almost entirely.
A More Favorable Metabolic Profile
As a result, far less of the parent drug is converted into the problematic DEO metabolite.
The transdermal route achieves a DEO-to-oxybutynin ratio of approximately 1.3 to 1. This dramatic shift in the metabolic profile is the key to its improved tolerability, with rates of dry mouth falling to levels comparable with a placebo.
Stable and Continuous Drug Levels
The patch provides a slow, continuous release of medication over 24 hours.
This avoids the sharp "peaks" and "troughs" in blood concentration that occur with oral dosing. Eliminating these peaks further reduces the risk of triggering concentration-dependent side effects.
Understanding the Trade-offs
While transdermal delivery solves the problem of systemic side effects, it introduces a different consideration.
Systemic Side Effects vs. Local Reactions
The primary advantage of the patch is the significant reduction in anticholinergic effects like dry mouth, which drops from as high as 93% with oral tablets to around 7% with the patch.
However, the most common side effect of the transdermal patch is a local one: application-site reactions, such as itching or redness. These are typically mild to moderate and are the main trade-off for improved systemic tolerability.
Efficacy Remains Intact
Crucially, this improvement in side effects does not compromise the drug's effectiveness.
Transdermal oxybutynin demonstrates comparable efficacy to oral agents in managing the symptoms of overactive bladder, such as reducing urinary urgency and frequency.
Making the Right Choice for Your Goal
Selecting the appropriate formulation depends on balancing efficacy, side effects, and patient preference.
- If your primary focus is minimizing systemic side effects: Transdermal oxybutynin is the clear choice, as it was specifically designed to avoid the metabolic pathway that causes dry mouth and constipation.
- If your primary focus is patient adherence: The patch's simpler, less frequent dosing schedule can improve compliance for many patients compared to taking multiple pills per day.
- If your primary focus is avoiding local skin irritation: An oral formulation, particularly an extended-release version, may be preferable despite the higher risk of systemic side effects.
Ultimately, understanding the metabolic journey of a drug is key to leveraging its delivery system for optimal patient outcomes.
Summary Table:
| Aspect | Oral Oxybutynin | Transdermal Oxybutynin |
|---|---|---|
| Key Metabolic Pathway | First-pass metabolism in gut/liver | Bypasses gut/liver, direct systemic absorption |
| DEO-to-Oxybutynin Ratio | ~11.9:1 (High) | ~1.3:1 (Low) |
| Common Side Effects | Dry mouth, constipation (systemic) | Application-site reactions (local) |
| Dry Mouth Incidence | Up to 93% | ~7% (placebo-like) |
| Efficacy | Effective for overactive bladder | Comparable efficacy to oral agents |
Partner with Enokon for Your Next Transdermal Patch Project
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon empowers healthcare and pharma distributors and brands with advanced drug delivery solutions. Our technical expertise ensures custom R&D and development tailored to your specific active ingredients, like oxybutynin, to optimize tolerability and efficacy.
Benefit from our manufacturing excellence to bring safer, more patient-friendly products to market. Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are potential challenges with transdermal patch therapy? Overcome Patient & Production Hurdles
- What precautions should be taken when using oxybutynin transdermal? Ensure Safe and Effective Treatment
- What is the generic name of the transdermal drug for depression? Selegiline Patch Explained
- How is a UV-Vis spectrophotometer applied to the clarity assessment of nanoemulgels? Quantify Formulation Stability
- What regulatory requirements apply to transdermal patches like MNPs in the United States? FDA Compliance Guide
- What special considerations exist for pediatric and geriatric patients using clonidine transdermal? Ensure Safe Use Across All Ages
- How should skin irritation from patches be managed? A Proactive Guide to Prevention and Care
- What monitoring is required while using estradiol patches? A Guide to Safe & Effective Hormone Therapy
















