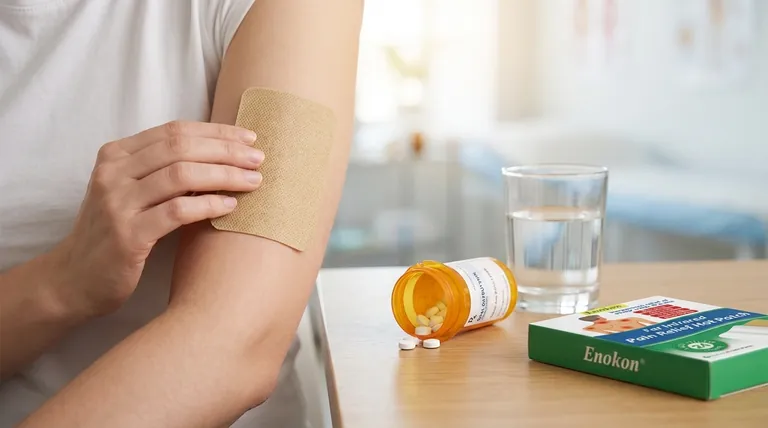
In simple terms, transdermal oxybutynin differs from the oral form by delivering the medication directly through the skin into the bloodstream. This bypasses the digestive system and initial processing by the liver, resulting in a more stable drug level, higher overall absorption, and a significant reduction in the metabolite often responsible for side effects like dry mouth.
The core advantage of the transdermal patch is not simply avoiding the stomach, but fundamentally altering how the body processes the medication. This shift dramatically reduces the formation of the metabolite linked to side effects while providing a steadier, more consistent therapeutic effect.
The Journey Through the Body: Oral vs. Transdermal
The method of delivery dictates the drug's path through the body, which has profound effects on its efficacy and tolerability.
The "First-Pass Effect" with Oral Oxybutynin
When you take an oral oxybutynin pill, it is absorbed from your gut and travels directly to the liver.
The liver acts as a processing center and immediately metabolizes a large portion of the drug before it can ever reach the rest of your body. This is known as the first-pass metabolism.
Bypassing the Liver with Transdermal Delivery
Transdermal oxybutynin, delivered via a patch or gel, is absorbed directly through the skin into the bloodstream.
This direct-to-bloodstream route completely bypasses the initial, aggressive metabolism by the liver, allowing the parent drug to circulate first and perform its function.
The Critical Role of Metabolites
Understanding the difference between the parent drug (oxybutynin) and what the body turns it into (metabolites) is key to understanding the two delivery methods.
What is N-desethyloxybutynin (DEO)?
When the liver metabolizes oxybutynin, its primary byproduct is an active metabolite called N-desethyloxybutynin (DEO).
While the parent oxybutynin is effective for bladder control, the DEO metabolite is strongly associated with the medication's common anticholinergic side effects, such as dry mouth and constipation.
Why the Oxybutynin-to-DEO Ratio Matters
The delivery method drastically changes the ratio of the parent drug to this problematic metabolite in your blood.
With oral administration, the ratio of oxybutynin to DEO is approximately 1 to 11.9. This means for every one part of the intended drug, you have nearly twelve parts of the side-effect-causing metabolite.
With transdermal delivery, this ratio is much more favorable, at approximately 1.3 to 1. The levels of the parent drug and the metabolite are nearly equal.
Understanding the Trade-offs and Delivery Dynamics
Beyond metabolites, the consistency of drug delivery and the amount absorbed are critical factors.
Bioavailability: Getting More from the Dose
Bioavailability refers to the percentage of a drug that successfully enters the bloodstream.
Because it avoids first-pass metabolism, transdermal oxybutynin has a very high bioavailability of around 80%. More of the drug in the patch gets to work in your body.
The Problem of Peaks and Troughs
Oral pills create a spike, or "peak," in drug and metabolite levels shortly after you take them, followed by a decline, or "trough," before the next dose.
These fluctuations can lead to more intense side effects at the peak and a potential loss of symptom control in the trough.
Consistent Delivery for Stable Efficacy
A transdermal patch provides a slow, continuous release of medication over several days.
This maintains a stable, steady-state concentration in the plasma, minimizing the peaks and troughs. The result is more consistent symptom management with a potentially lower burden of side effects.
Making the Right Choice for Your Goal
- If your primary focus is minimizing side effects: The transdermal route is often preferred, as it produces significantly lower levels of the DEO metabolite associated with dry mouth and constipation.
- If your primary focus is consistent, 24/7 symptom control: Transdermal delivery provides a steady level of medication, avoiding the peaks and troughs of oral dosing that can lead to breakthrough symptoms.
- If you have experienced side effects with oral oxybutynin: The transdermal form offers a distinct metabolic profile that may allow you to tolerate the medication effectively.
Understanding these metabolic and delivery differences empowers you to have a more informed discussion with your healthcare provider about the optimal treatment strategy for your needs.
Summary Table:
| Feature | Oral Oxybutynin | Transdermal Oxybutynin (TDOXY) |
|---|---|---|
| Route of Administration | Swallowed (GI Tract) | Patch/Gel (Through the Skin) |
| First-Pass Liver Metabolism | High | Bypassed |
| Oxybutynin-to-DEO Ratio | ~1 : 11.9 | ~1.3 : 1 |
| Bioavailability | Lower | High (~80%) |
| Drug Delivery | Peaks & Troughs | Steady, Continuous |
| Primary Benefit | Convenience | Fewer Side Effects (e.g., Dry Mouth), Consistent Control |
Ready to develop a superior transdermal patch?
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise for custom R&D and development to create products that offer patients better efficacy and tolerability.
Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Natural Herbal Wormwood Patch Pain Plaster
People Also Ask
- What are the functions of glutaraldehyde and osmium tetroxide in sample prep? Expert Insights for Skin Morphology
- What is the function of a rotary evaporator in green tea transfersome prep? Expert Guide to Lipid Thin Film Formation
- What is the technical logic behind selecting semipermeable barrier membranes? Optimize In Vitro Drug Release Accuracy
- What should be done if severe side effects occur while using selegiline skin patch? Recognize a Hypertensive Crisis
- What are the pharmacokinetic properties of the transdermal patch form of this medication? Key Benefits of Steady 24-Hour Delivery
- What factors can affect transdermal drug absorption in pets? Key Considerations for Effective Treatment
- What is the concern regarding the use of transdermal long-acting β2 agonist patches in Japan? Risks of Off-Label Use Explained
- What historical milestone marked the commercial use of transdermal patches? The 1979 Breakthrough That Revolutionized Drug Delivery
















