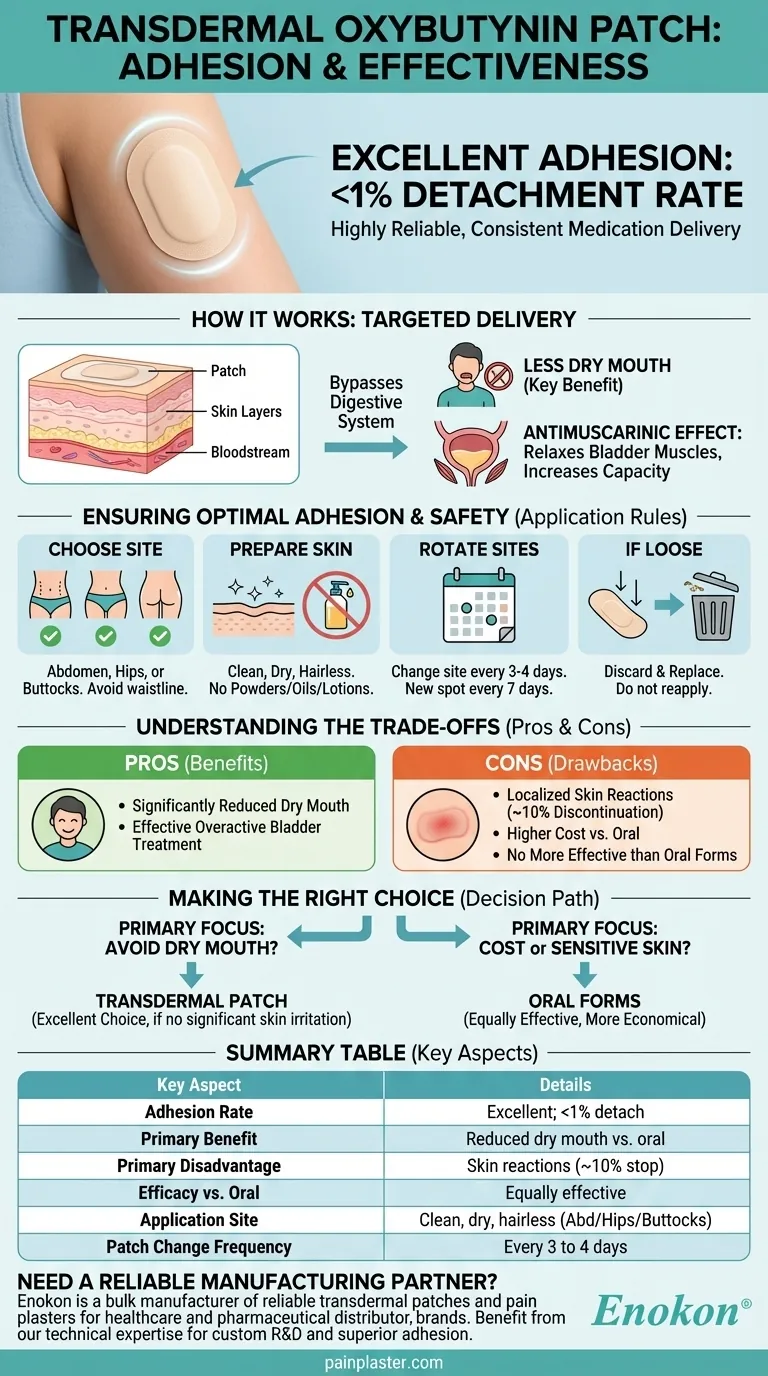
In short, patch adhesion for transdermal oxybutynin is excellent. The data shows that the patch is highly reliable, with fewer than 1 percent of patches detaching either partially or completely during use. This high rate of adhesion ensures consistent medication delivery for patients who use it correctly.
The core issue isn't whether the oxybutynin patch will stick—it almost certainly will. The real decision hinges on balancing its primary benefit of reduced dry mouth against its significant potential for skin irritation and higher cost compared to oral alternatives.

How the Oxybutynin Patch Works
The transdermal patch is designed to treat an overactive bladder by delivering oxybutynin directly through the skin into the bloodstream.
The Antimuscarinic Effect
Oxybutynin is an antimuscarinic agent. It works by relaxing the smooth muscles of the bladder.
This action decreases uncontrolled bladder contractions, which in turn increases the bladder's capacity, delays the urge to urinate, and alleviates the primary symptoms of an overactive bladder.
The Purpose of Transdermal Delivery
Delivering the medication through a patch allows it to bypass the digestive system. This method of delivery is a key reason why it causes less dry mouth than the oral tablet forms.
Ensuring Optimal Patch Adhesion and Safety
While the patch's adhesive is effective, proper application is critical for ensuring it works as intended and for minimizing skin issues.
Choose the Correct Application Site
The patch should be applied to clean, dry, and preferably hairless skin on the abdomen (stomach), hips, or buttocks.
Avoid placing it on the waistline where clothing might rub against it excessively. Also, ensure the patch is placed under clothing to avoid sun exposure.
Prepare the Skin Properly
The application area must be free of any powders, oils, or lotions, as these can interfere with the adhesive and prevent the patch from sticking securely.
Rotate Application Sites Consistently
To prevent skin irritation, you must rotate the application site. A new patch should not be placed on the same spot of skin for at least seven days. The patch is typically changed every 3 to 4 days.
If a Patch Comes Loose
If a patch falls off, it should be discarded and replaced with a new one applied to a different site. Do not attempt to reapply a patch that has lost its adhesion.
Understanding the Trade-offs
The oxybutynin patch is a valuable tool, but it is not without its drawbacks. An objective assessment of its pros and cons is essential.
Key Advantage: Reduced Dry Mouth
The most significant benefit of the patch over oral oxybutynin is a lower incidence of dry mouth, a common and often bothersome side effect of the tablets.
Primary Disadvantage: Skin Reactions
Despite good adhesion, localized skin reactions are the patch's primary drawback. These reactions are significant enough to cause approximately 10 percent of patients to discontinue use.
Efficacy and Cost
Clinical evidence shows the patch is no more effective than the short- or long-acting oral forms of oxybutynin. It simply offers a different side-effect profile.
Furthermore, the patch is generally more expensive than the oral medication alternatives.
Making the Right Choice for Your Goal
Selecting the right form of oxybutynin depends entirely on your personal priorities and medical history.
- If your primary focus is avoiding side effects like dry mouth: The transdermal patch is an excellent choice, provided you do not experience significant skin irritation.
- If your primary focus is cost or you have sensitive skin: The oral forms are equally effective at treating overactive bladder and are more economical, though they carry a higher risk of dry mouth.
Ultimately, understanding these distinct profiles empowers you to work with your healthcare provider to select the treatment that best fits your needs.
Summary Table:
| Key Aspect | Details for Oxybutynin Patch |
|---|---|
| Adhesion Rate | Excellent; <1% of patches detach |
| Primary Benefit | Significantly reduced dry mouth vs. oral forms |
| Primary Disadvantage | Localized skin reactions (~10% discontinuation rate) |
| Efficacy vs. Oral | Equally effective at treating overactive bladder |
| Application Site | Clean, dry, hairless skin on abdomen, hips, or buttocks |
| Patch Change Frequency | Every 3 to 4 days |
Need a reliable manufacturing partner for your transdermal patches?
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise for custom R&D and development to ensure your products, like an oxybutynin patch, feature superior adhesion and consistent drug delivery.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- What are the benefits of using Chinese pain patches? Natural Relief with Traditional Healing
- How is the heat-sealable ethylene-vinyl acetate (EVA) membrane utilized within transdermal patches? Master Precision Release
- What precautions should be taken when using topical patches for back pain? Ensure Safe & Effective Pain Relief
- What are the roles of polyethylene backing and polyester liner in CBD patches? Key Roles in Transdermal Stability
- Why specify equipment parameters in transdermal patch documentation? Ensure Scientific Reliability & Batch Consistency
- What is the function of Propylene glycol in the preparation of transdermal patches? The Key to Flexible, Durable Films.
- What is the primary function of the backing layer in a TDDS? Master Transdermal Patch Integrity and Delivery Efficiency
- How does the medication in menthol and methyl salicylate patches work? | Pain Relief Explained

















