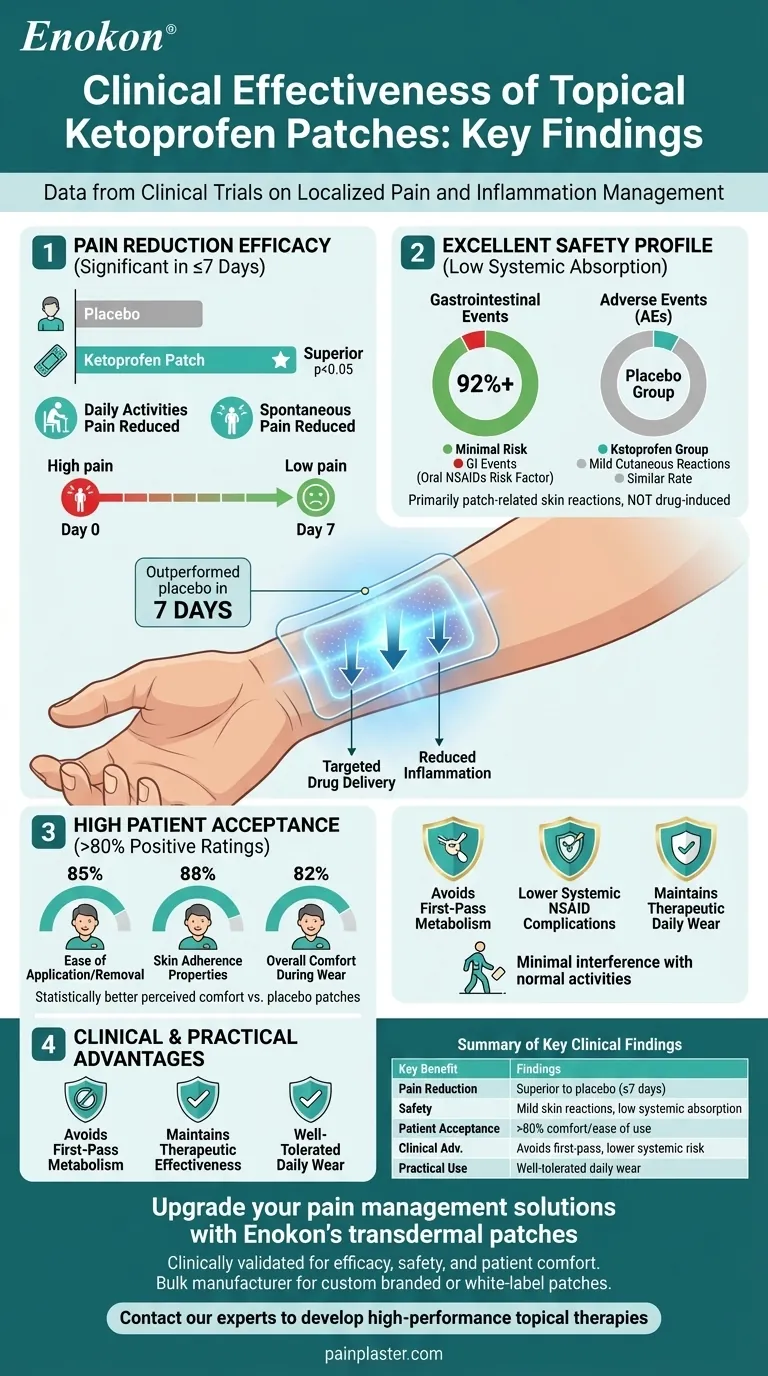
Topical ketoprofen patches demonstrated significant effectiveness in clinical trials for managing localized pain and inflammation. The patch outperformed placebo in reducing both activity-related and spontaneous pain after just 7 days of treatment, while maintaining an excellent safety profile with primarily mild cutaneous reactions. Over 80% of patients reported high satisfaction with patch comfort and usability, positioning it as a patient-friendly alternative to oral NSAIDs with minimal systemic side effects.
Key Points Explained:
-
Pain Reduction Efficacy
- Consistently superior to placebo in clinical trials for:
- Pain during daily activities (p<0.05)
- Spontaneous pain at rest
- Significant improvements observed within 7 days of treatment
- Multiple trial results showed reproducible effectiveness across study populations
- Consistently superior to placebo in clinical trials for:
-
Safety Profile
- Adverse events were primarily cutaneous (skin-related) and:
- Occurred at similar rates in both ketoprofen and placebo groups
- Suggested to be patch-related rather than drug-induced
- Low systemic absorption demonstrated by:
- Gastrointestinal adverse events <8%
- No significant difference between active and placebo groups
- Adverse events were primarily cutaneous (skin-related) and:
-
Patient Acceptance
- Received >80% positive ratings for:
- Ease of application/removal
- Skin adherence properties
- Overall comfort during wear
- Statistically better perceived comfort versus placebo patches
- Received >80% positive ratings for:
-
Clinical Advantages
- Targeted therapy avoids first-pass metabolism
- Lower risk of systemic NSAID complications compared to oral formulations
- Maintains therapeutic effectiveness for localized inflammation
-
Practical Considerations
- Daily wear protocol well-tolerated
- Minimal interference with normal activities
- Favorable risk-benefit ratio for chronic use cases
The combination of demonstrated efficacy, safety, and patient preference makes these patches a compelling option for clinicians managing localized musculoskeletal pain, particularly for patients who cannot tolerate oral NSAIDs or require prolonged anti-inflammatory therapy.
Summary Table:
| Key Benefit | Clinical Trial Findings |
|---|---|
| Pain Reduction | Superior to placebo in activity-related & spontaneous pain (significant in ≤7 days) |
| Safety Profile | Primarily mild skin reactions; low systemic absorption (GI events <8%) |
| Patient Acceptance | >80% satisfaction for comfort, adhesion, and ease of use |
| Clinical Advantages | Avoids first-pass metabolism; lower systemic risks vs. oral NSAIDs |
| Practical Use | Well-tolerated for daily wear; minimal activity interference |
Upgrade your pain management solutions with Enokon's transdermal patches — clinically validated for efficacy, safety, and patient comfort. As a bulk manufacturer specializing in custom transdermal drug delivery systems, we offer:
- Tailored R&D for branded or white-label patches
- Proven formulations with reliable adhesion & drug release
- Regulatory-compliant production for healthcare/pharma partners
Contact our experts today to develop high-performance topical therapies for your patients.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















