Transdermal patch toxicity is monitored through a combination of preclinical skin irritation studies, predictive modeling, and post-market surveillance. Primary methods include the Primary Irritation Index (PII) test to evaluate localized skin reactions like erythema and edema, alongside computational models analyzing drug-skin interactions. Proper disposal protocols are critical to prevent secondary exposure risks. These layered approaches ensure safety from development through real-world use.
Key Points Explained:
-
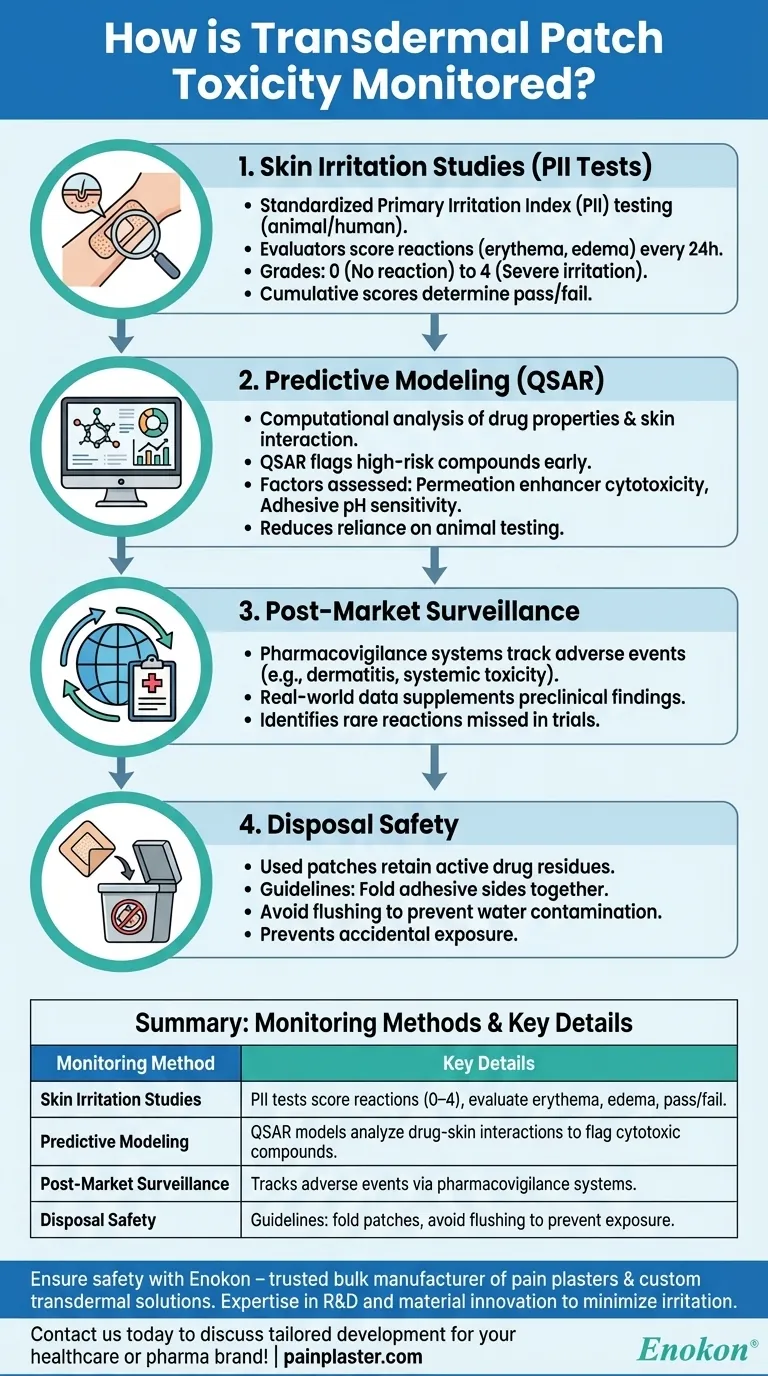
Skin Irritation Studies (PII Tests)
- The transdermal patch undergoes standardized Primary Irritation Index (PII) testing, where patches are applied to animal or human skin under controlled conditions.
- Evaluators score reactions (redness, swelling) at 24-hour intervals, with grades:
- 0 (no reaction) to 4 (severe irritation).
- Cumulative scores determine pass/fail thresholds for further development.
-
Predictive Modeling
- Computational tools analyze drug properties (e.g., solubility, molecular weight) and their interaction with skin layers.
- Models like QSAR (Quantitative Structure-Activity Relationship) flag high-risk compounds early, reducing reliance on animal testing.
- Factors assessed:
- Chemical permeation enhancers’ cytotoxicity.
- pH sensitivity of adhesive layers.
-
Post-Market Surveillance
- Pharmacovigilance systems track adverse events (e.g., contact dermatitis, systemic toxicity) reported by healthcare providers or patients.
- Real-world data supplements preclinical findings, identifying rare reactions missed in trials.
-
Disposal Safety
- Used patches retain active drug residues; improper disposal risks accidental exposure (e.g., children/pets).
- Guidelines include:
- Folding adhesive sides together before disposal.
- Avoiding flushing to prevent water contamination.
-
Emerging Technologies
- Microneedle arrays and biodegradable materials aim to reduce irritation risks while enhancing drug delivery precision.
By integrating these strategies, manufacturers balance efficacy with patient safety—ensuring patches meet regulatory standards while minimizing harm. Have you considered how material innovations might further reduce irritation in future designs?
Summary Table:
| Monitoring Method | Key Details |
|---|---|
| Skin Irritation Studies | PII tests score reactions (0–4) to evaluate erythema, edema, and pass/fail thresholds. |
| Predictive Modeling | QSAR models analyze drug-skin interactions to flag cytotoxic compounds early. |
| Post-Market Surveillance | Tracks adverse events (e.g., dermatitis) via pharmacovigilance systems. |
| Disposal Safety | Guidelines include folding patches and avoiding flushing to prevent exposure. |
Ensure your transdermal patches meet safety standards with Enokon—your trusted bulk manufacturer of reliable pain plasters and custom transdermal solutions. Our expertise in R&D and material innovation helps minimize irritation risks while maximizing efficacy. Contact us today to discuss tailored development for your healthcare or pharma brand!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained












