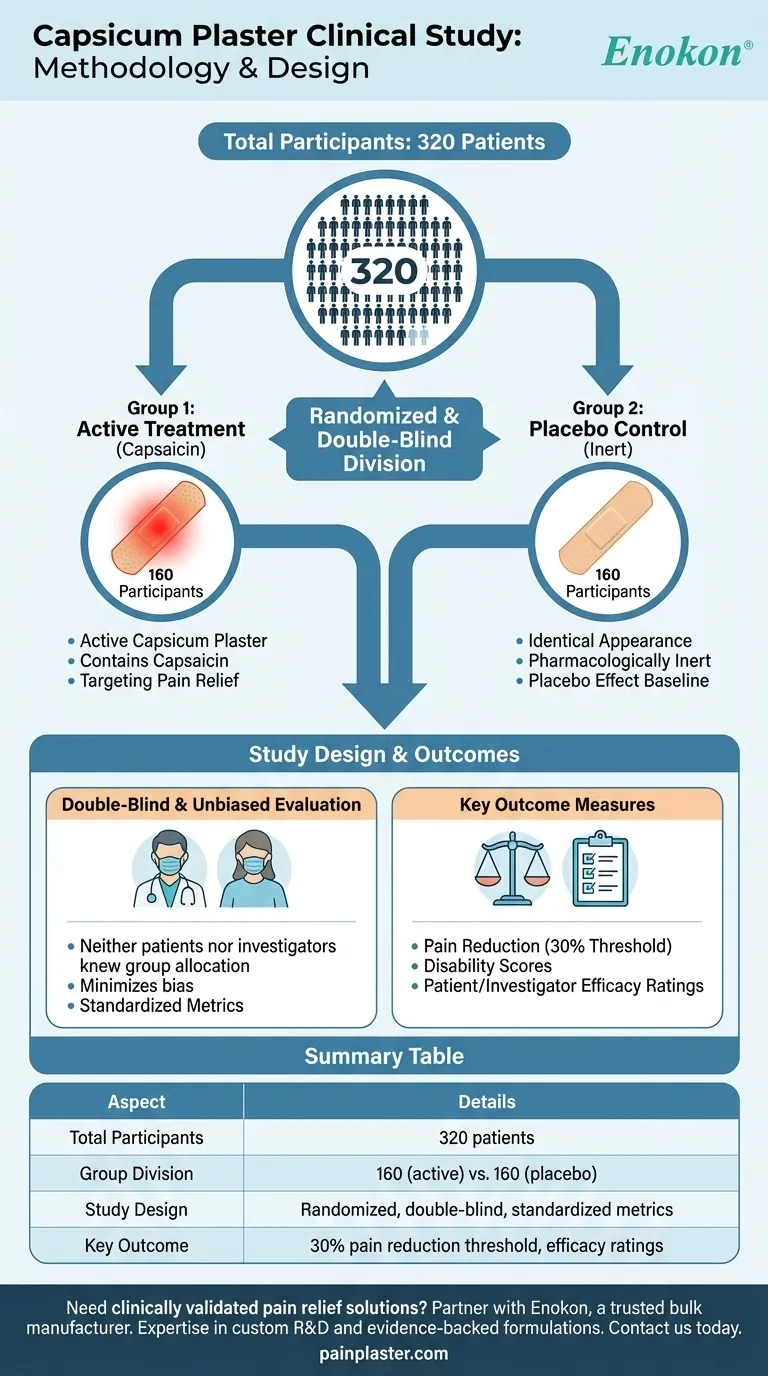
The study involved a total of 320 patients who were randomly divided into two equal groups of 160 participants each. One group received treatment with an active capsicum plaster, while the other group was given a placebo plaster. This randomized, double-blind setup ensured unbiased evaluation of the plaster's efficacy in managing pain, with outcomes measured using standardized scales and patient-reported metrics.

Key Points Explained:
-
Total Participants
- The study enrolled 320 patients, a sample size large enough to provide statistically significant results while maintaining manageable clinical trial logistics.
-
Group Division
- Randomized allocation: Patients were split into two groups of 160 each to ensure balanced comparison.
-
Treatment vs. placebo:
- Group 1: Active capsicum plaster (containing capsaicin).
- Group 2: Placebo plaster (identical in appearance but pharmacologically inert).
- Randomization minimized bias in demographic or clinical characteristics between groups.
-
Study Design
- Double-blind: Neither patients nor investigators knew which plaster was active, reducing placebo effect and observer bias.
- Outcome measures: Included pain reduction (30% threshold), disability scores, and patient/investigator efficacy ratings.
-
Why This Matters
- Equal group sizes and randomization strengthen the reliability of conclusions about the plaster’s effectiveness.
- The design mirrors real-world clinical decision-making, where clear evidence of superiority over placebo is critical for adoption.
This structured approach highlights how rigorous methodology supports evidence-based recommendations for pain management tools like capsicum plaster.
Summary Table:
| Aspect | Details |
|---|---|
| Total Participants | 320 patients |
| Group Division | 160 patients (active capsicum plaster) vs. 160 (placebo) |
| Study Design | Randomized, double-blind, with standardized pain/disability metrics |
| Key Outcome | 30% pain reduction threshold, efficacy ratings by patients/investigators |
Need clinically validated pain relief solutions? Partner with Enokon, a trusted bulk manufacturer of transdermal patches and pain plasters. Our expertise in custom R&D ensures evidence-backed formulations tailored to your brand or distribution needs. Contact us today to discuss scalable, compliant solutions for your market.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What should be done if a dose of the Capsicum HOT PATCH Adhesive Patch is missed? Follow These Steps for Safe Use
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does the active ingredient Capsicum in the Heat Patch work? A Dual-Action Path to Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
















