For healthcare providers, patients using transdermal selegiline must be monitored frequently, particularly at the beginning of treatment. This close follow-up is essential not only to assess the therapeutic response but also to manage dose adjustments and monitor for specific risks associated with this class of medication. Patients must be instructed to keep all scheduled appointments to ensure their safety and the drug's efficacy.
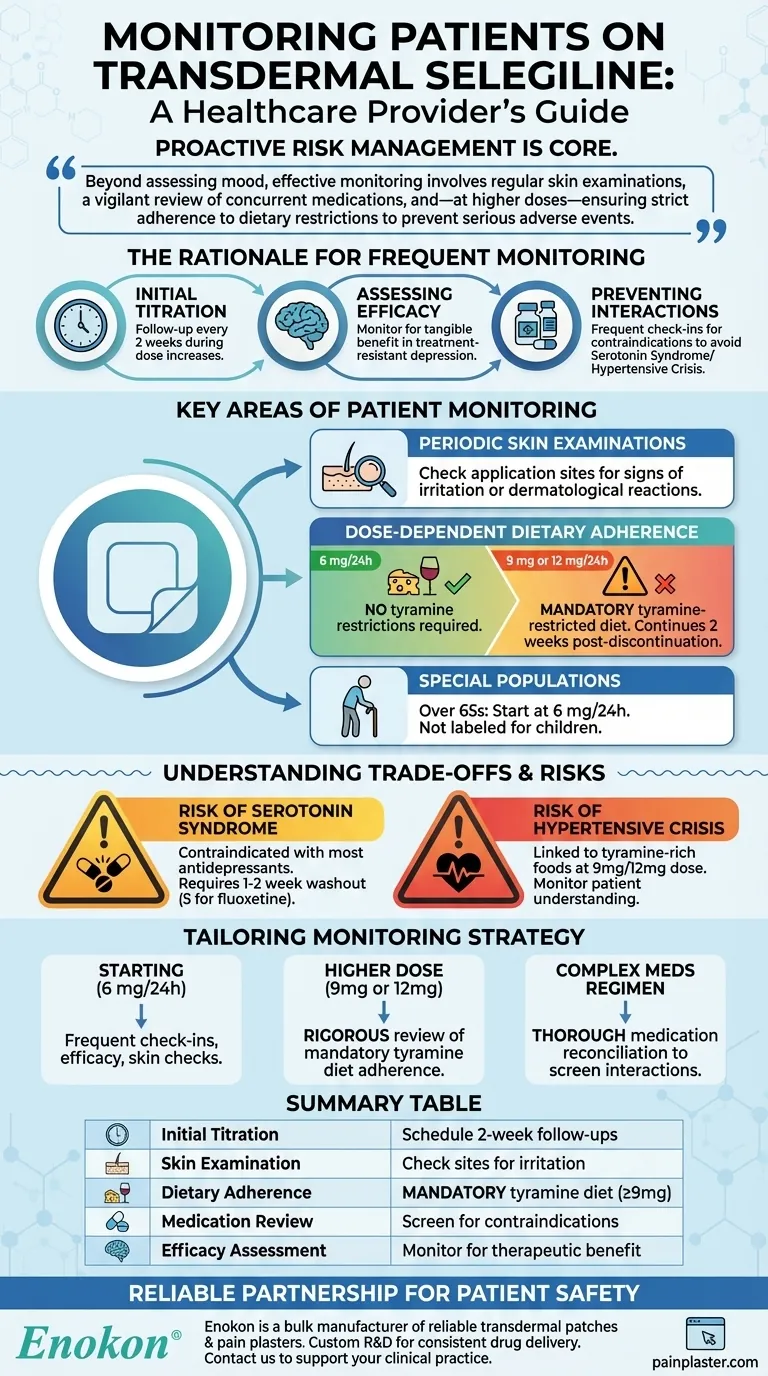
The core principle of monitoring transdermal selegiline is proactive risk management. Beyond assessing mood, effective monitoring involves regular skin examinations, a vigilant review of all concurrent medications, and—at higher doses—ensuring strict adherence to dietary restrictions to prevent serious adverse events.

The Rationale for Frequent Monitoring
Close and consistent patient contact is non-negotiable when prescribing transdermal selegiline. The need for this is rooted in the drug's mechanism, potential for interactions, and the typical patient population for whom it is prescribed.
The Initial Titration Phase
The starting dose is typically 6 mg/24 hours. Dosage increases of 3 mg may occur every two weeks, creating a natural schedule for frequent follow-up appointments during the initial phase of treatment.
Assessing Efficacy
Transdermal selegiline is often reserved for patients with treatment-resistant or atypical depression. This context requires diligent monitoring to confirm that this potent medication is providing a tangible benefit.
Preventing Dangerous Interactions
As a monoamine oxidase inhibitor (MAOI), selegiline is contraindicated with many other antidepressants and medications. Frequent check-ins allow you to review the patient's complete medication list, including over-the-counter drugs and supplements, to prevent serotonin syndrome or a hypertensive crisis.
Key Areas of Patient Monitoring
Effective oversight goes beyond a simple check-in. It requires a focused assessment of several key areas at each appointment.
Periodic Skin Examinations
The transdermal patch delivery system necessitates periodic skin examinations. You must check the application sites for signs of irritation or other dermatological reactions.
Dose-Dependent Dietary Adherence
This is a critical distinction. At the 6 mg/24 hours dose, no tyramine dietary restrictions are required.
However, once the dose is increased to 9 mg or 12 mg/24 hours, the patient must follow a tyramine-restricted diet. This monitoring includes patient education, reinforcement, and continues for two weeks after discontinuation.
Special Population Considerations
While studies have not shown specific problems in the elderly, the recommended dose for patients over 65 is the starting dose of 6 mg/24 hours. The medication is not labeled for use in children.
Understanding the Trade-offs and Risks
Trust and safety are built on a clear understanding of a medication's potential downsides. Selegiline is highly effective for the right patient but carries significant risks if not managed properly.
The Risk of Serotonin Syndrome
Combining selegiline with most other antidepressants is contraindicated due to the high risk of serotonin syndrome. A "washout" period of 1-2 weeks (or 5 weeks for fluoxetine) is required when switching from a contraindicated drug.
The Risk of Hypertensive Crisis
This severe spike in blood pressure is primarily linked to consuming tyramine-rich foods while on the 9 mg or 12 mg dose. Monitoring patient understanding and adherence to the diet is a primary safety measure.
Cost and Place in Therapy
Transdermal selegiline is an expensive option. Its use in patients who have not responded to other treatments underscores the importance of careful monitoring to ensure the benefits justify the cost and complexity.
Tailoring Monitoring to Your Patient's Needs
Your monitoring strategy should adapt to the patient's current dosage and situation. Use these guidelines to structure your approach.
- If your patient is starting treatment (6 mg/24h): Focus on frequent check-ins (e.g., aligned with the two-week titration schedule) to assess initial efficacy, side effects, and conduct baseline skin checks.
- If your patient is on a higher dose (9 mg or 12 mg/24h): Your monitoring must include a rigorous review of their understanding and adherence to the mandatory tyramine-restricted diet.
- If your patient has a complex medication regimen: Prioritize a thorough medication reconciliation at every visit to screen for potentially interacting drugs or supplements.
Ultimately, proactive and comprehensive monitoring is the key to leveraging the therapeutic power of transdermal selegiline safely.
Summary Table:
| Monitoring Aspect | Key Action Points |
|---|---|
| Initial Titration | Schedule follow-ups every 2 weeks during dose increases. |
| Skin Examination | Check application sites periodically for irritation. |
| Dietary Adherence | Mandatory tyramine-restricted diet at doses of 9 mg/24h or higher. |
| Medication Review | Screen for contraindicated drugs/supplements at every visit to prevent serotonin syndrome. |
| Efficacy Assessment | Monitor for therapeutic benefit, especially in treatment-resistant depression. |
Ensure your patients' safety with reliable transdermal delivery systems.
As a healthcare provider managing complex treatments like transdermal selegiline, you need a manufacturing partner you can trust.
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. We understand the critical importance of consistent drug delivery and patient safety. Our technical expertise supports custom R&D and development, ensuring the patches you use meet the highest standards of quality and performance.
Let us help you enhance patient outcomes. Contact our team today to discuss how our transdermal solutions can support your clinical practice.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How does a magnetic stirring device influence transdermal diffusion? 4 Keys to Accurate Permeability Data
- What specialized R&D support is available for pectin/gelatin transdermal patches? Master Natural Polymer Manufacturing
- What is the critical role of a precision film coater in the production of transdermal patches? Master Precise Drug Dosing
- How should nitroglycerin patches be applied? Ensure Correct Use for Maximum Effectiveness
- How should the Signal Relief patch be used for upper backache relief? Master Precise Placement for Maximum Relief
- How should used Butrans patches be disposed of? Safe Disposal Guide to Prevent Accidental Exposure
- How did the ketoprofen patch compare to previous studies on similar treatments? Key Findings on Efficacy & Convenience
- What is a pain patch and how does it work? Targeted Relief Through Transdermal Technology

















