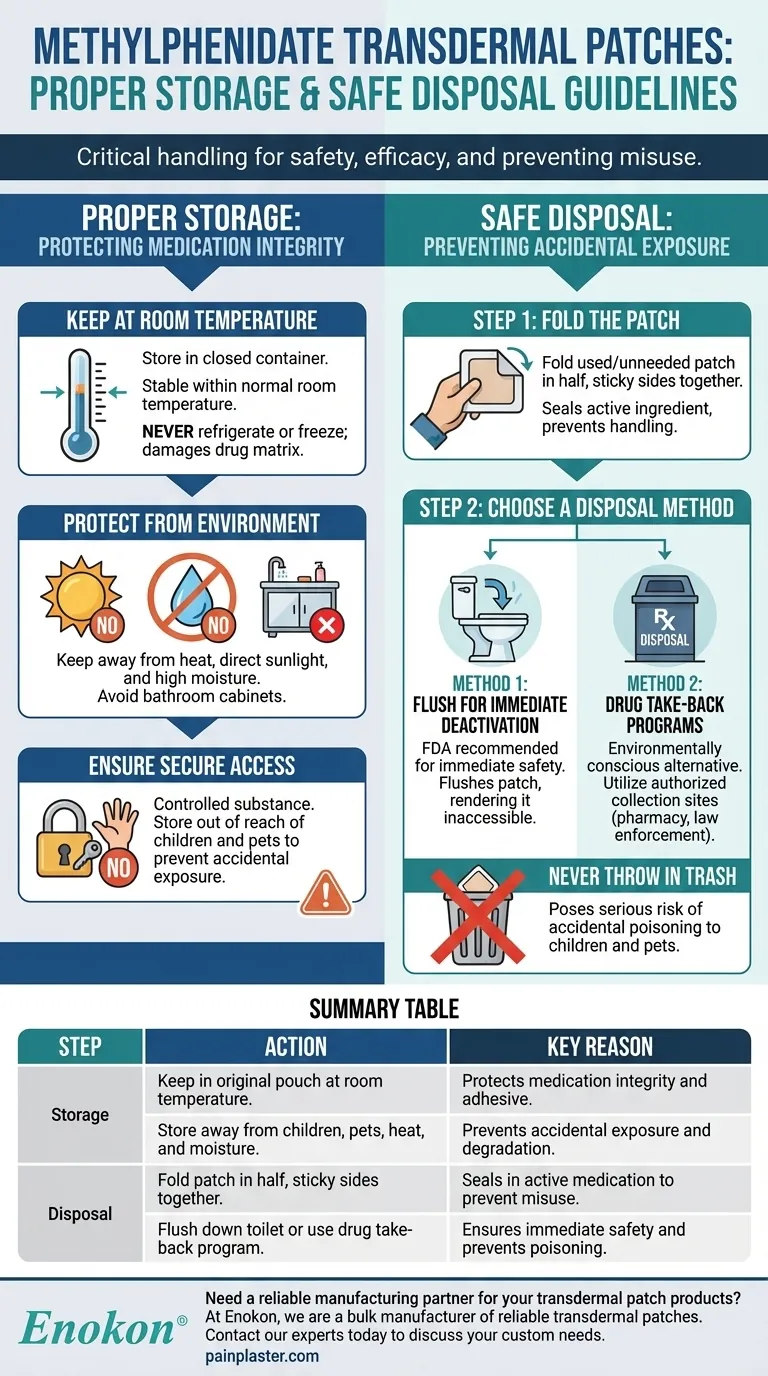
Proper handling is critical for both safety and effectiveness. Methylphenidate transdermal patches must be stored at room temperature in a closed container, kept away from heat, moisture, and direct light. For disposal, the used or unneeded patch should be folded in half with the sticky side in and either flushed down the toilet or taken to an authorized drug take-back location.
The strict storage and disposal guidelines for methylphenidate patches are designed to protect the medication's integrity and, more importantly, to prevent accidental exposure or intentional misuse of this potent, controlled substance.

Proper Storage: Protecting Medication Integrity
Correct storage ensures the patch delivers the right amount of medication consistently and safely.
Keep at Room Temperature
The medication is designed to be stable within a normal room temperature range.
Extreme temperatures can alter the patch's properties. Never refrigerate or freeze the patches, as this can damage the drug matrix and adhesive layer.
Protect from Environmental Factors
Store the patches in their original sealed pouch or a closed container until you are ready to use one.
Keep them away from sources of heat, direct sunlight, and high moisture, such as a bathroom medicine cabinet, which can degrade the medication and compromise the adhesive.
Ensure Secure and Safe Access
Methylphenidate is a controlled substance with a potential for misuse.
Always store the patches in a safe place that is out of the reach of children and pets to prevent accidental contact or ingestion.
Safe Disposal: Preventing Accidental Exposure
Even a used patch contains a significant amount of active medication. Improper disposal poses a serious risk to others.
The Critical First Step: Folding the Patch
Whether the patch is used, expired, or no longer needed, the first step is always the same.
Fold the patch in half so that the sticky, medication-containing sides press firmly together. This seals the active ingredient and makes it much more difficult to handle or misuse.
Method 1: Flushing for Immediate Deactivation
The FDA recommends flushing certain powerful medicines to prevent immediate danger from accidental exposure. Methylphenidate is on this list.
Flushing the folded patch down the toilet ensures it is immediately rendered inaccessible and cannot be accidentally retrieved from the trash by a child, pet, or anyone else.
Method 2: Drug Take-Back Programs
As an alternative, you can take the folded patches to an authorized drug take-back location.
These programs are designed for the safe and environmentally conscious disposal of pharmaceuticals. Check with your local pharmacy or law enforcement agency for collection sites.
Understanding the Risks and Trade-offs
The specific disposal instructions are in place to prioritize human safety over other concerns.
The Danger of Household Trash
Never throw methylphenidate patches in the trash.
Even a used patch contains enough medication to be dangerous or even fatal if ingested by a child or pet. Discarding it in the trash creates a direct and avoidable risk of poisoning and misuse.
Flushing vs. Environmental Concerns
Health authorities have determined that the immediate risk of accidental exposure or overdose from these specific drugs outweighs the potential trace amounts that may enter the water supply.
While take-back programs are an excellent option, the recommendation to flush is a pragmatic safety measure designed to prevent immediate harm when a take-back program is not readily available.
The Risk of Heat Exposure
Avoiding heat is a critical safety rule for both storage and use.
Applying a direct heat source (like a heating pad or electric blanket) over a worn patch can cause the medication to be released too quickly, leading to a dangerous overdose. Similarly, storing patches in a hot car can damage them.
Applying This to Your Routine
Your choice of disposal method depends on balancing immediate safety with environmental considerations.
- If your primary focus is preventing immediate misuse or accidental exposure: The FDA-recommended method of folding the patch and flushing it down the toilet is the most direct and secure approach.
- If your primary focus is environmental impact and a take-back program is accessible: Using an authorized drug take-back location is the preferred alternative for keeping pharmaceuticals out of the ecosystem.
By following these precise handling procedures, you ensure the medication works as intended while protecting your family and community.
Summary Table:
| Step | Action | Key Reason |
|---|---|---|
| Storage | Keep in original pouch at room temperature. | Protects medication integrity and adhesive. |
| Storage | Store away from children, pets, heat, and moisture. | Prevents accidental exposure and degradation. |
| Disposal | Fold patch in half, sticky sides together. | Seals in active medication to prevent misuse. |
| Disposal | Flush down toilet or use drug take-back program. | Ensures immediate safety and prevents poisoning. |
Need a reliable manufacturing partner for your transdermal patch products? Proper handling begins with a high-quality, consistently manufactured product. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise for custom R&D and development to ensure your products meet the highest standards of safety and efficacy from production to patient use.
Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief













