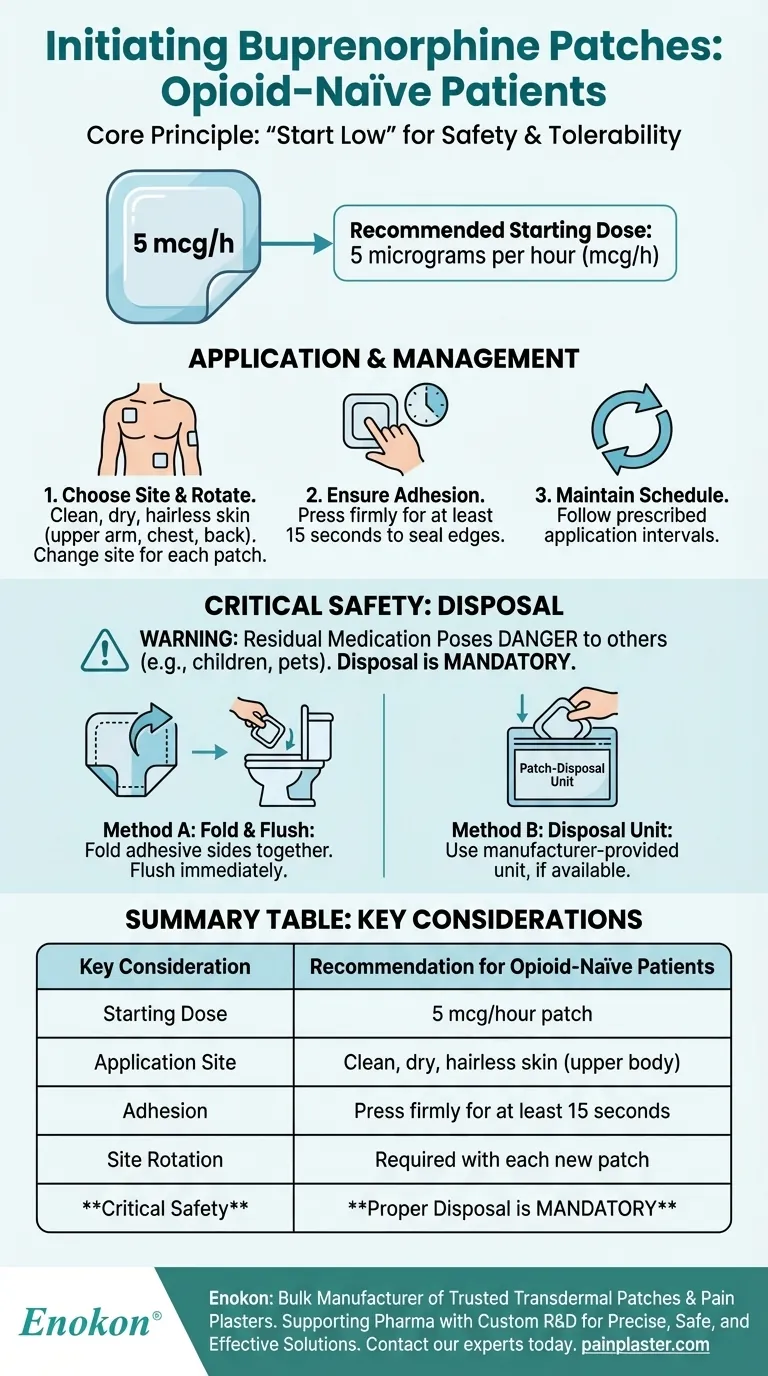
For patients who are opioid-naïve, the buprenorphine patch is initiated at the lowest available strength to ensure safety and tolerability. The recommended starting dose is 5 micrograms per hour (mcg/h).
The core principle for initiating an opioid-naïve patient on the buprenorphine patch is to "start low." Beginning with the 5 mcg/h dose minimizes risks while allowing you to assess the patient's response. Proper education on application and disposal is just as critical as selecting the right dose.
The Foundation: Why a Low Dose is Non-Negotiable
Initiating any opioid requires a cautious approach, especially in individuals without prior exposure. The strategy for an opioid-naïve patient is fundamentally different from that for a patient who has developed tolerance.
The Standard Starting Dose
The 5 mcg/h patch is the designated starting point for opioid-naïve patients. This low dose delivers a steady, minimal amount of medication to gauge the patient's sensitivity and response.
Contrasting with Opioid-Tolerant Patients
This contrasts sharply with patients already taking opioids. For them, initiation requires calculating their total daily oral morphine equivalent to convert them to an appropriate, and typically much higher, starting patch strength.
Critical Steps for Application and Management
Proper administration is essential for the patch to deliver its intended therapeutic effect safely and consistently. Counsel your patients on these precise steps.
Choosing the Right Application Site
The patch must be applied to a clean, dry, and relatively hairless area of skin. Ideal locations are on the upper outer arm, upper chest, upper back, or the side of the chest.
Ensuring Proper Adhesion
After removing the patch from its sealed sachet, apply it immediately to the selected site. Press down firmly with the palm of your hand for at least 15 seconds to ensure the edges are completely sealed against the skin.
The Importance of Site Rotation
To prevent skin irritation, instruct the patient to choose a different site for each new patch application.
Understanding the Trade-offs and Safety Protocols
While effective, buprenorphine is a potent opioid. Strict adherence to safety protocols, particularly regarding disposal, is critical to prevent accidental exposure and harm.
The Risk of Residual Medication
Even after it has been worn, a used patch contains a significant amount of buprenorphine. Improper disposal can pose a serious danger to children, pets, or others who might come into contact with it.
Mandated Disposal Methods
Patients must be instructed on how to dispose of the patch safely. There are two primary methods:
- Fold and Flush: Fold the used patch in half so the adhesive sides stick firmly together, and then flush it down the toilet.
- Disposal Unit: Use the special Patch-Disposal Unit provided by the manufacturer, if available.
A Checklist for Safe Initiation
Use these points to guide your approach based on the specific needs of your patient.
- If your primary focus is patient safety: Always initiate an opioid-naïve patient at the 5 mcg/h dose and provide clear education on potential side effects.
- If your primary focus is consistent medication delivery: Emphasize the technique of pressing the patch firmly for 15 seconds on a clean, hairless, upper-body site.
- If your primary focus is preventing accidental exposure: Stress the importance of the "fold and flush" method or using the provided disposal unit for every used patch.
Following this structured approach ensures that therapy is initiated safely, effectively, and with minimal risk.
Summary Table:
| Key Consideration | Recommendation for Opioid-Naïve Patients |
|---|---|
| Starting Dose | 5 mcg/hour patch |
| Application Site | Clean, dry, hairless skin (upper arm, chest, back) |
| Adhesion | Press firmly for at least 15 seconds |
| Site Rotation | Required with each new patch to prevent irritation |
| Critical Safety | Proper disposal (fold & flush or use disposal unit) is mandatory |
Ensure patient safety and effective pain management with reliable transdermal patches. As Enokon, a bulk manufacturer of trusted transdermal patches and pain plasters, we provide the foundational technology for healthcare and pharma brands. Our technical expertise supports custom R&D for developing precise, safe, and effective transdermal solutions. Contact our experts today to discuss how we can support your product development needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief














