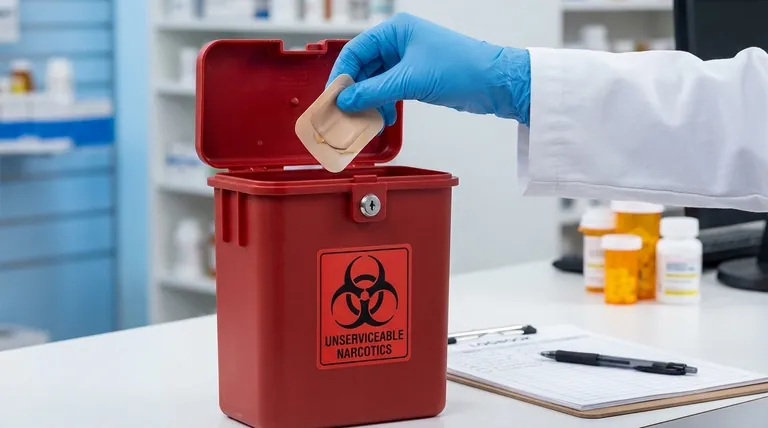
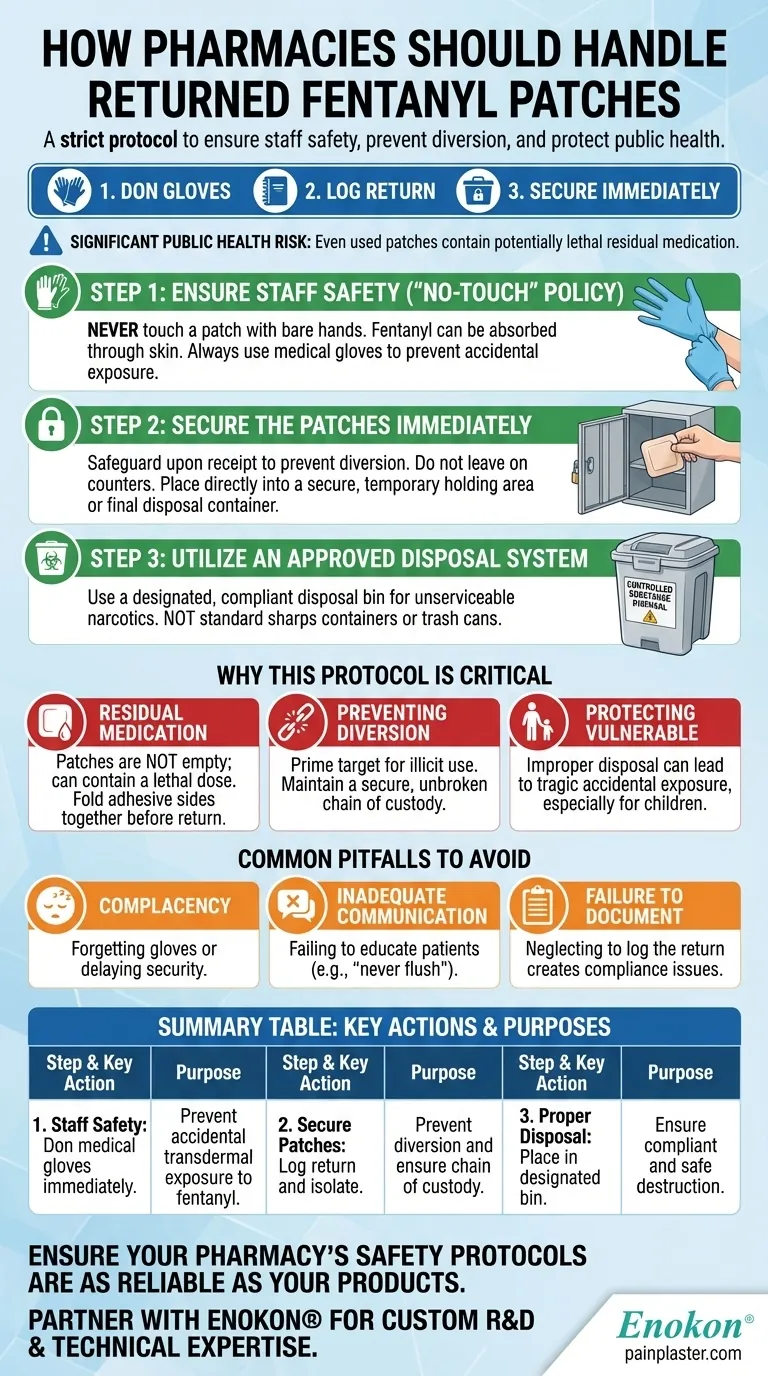
To properly handle returned fentanyl patches, pharmacy staff must follow a strict safety and security protocol. Personnel should immediately don medical gloves to avoid direct contact, log the return, and secure the patch in a designated bin for unserviceable narcotics to prevent diversion or accidental exposure.
Returned fentanyl patches pose a significant public health risk because even used patches contain a potentially lethal amount of medication. A pharmacy's core responsibility is to establish a secure, unbroken chain of custody from the moment a patient returns a patch to its final destruction.
The Core Protocol: From Receipt to Disposal
The procedure for handling returned controlled substances like fentanyl is designed to protect staff, the public, and the environment. Each step is a critical link in a chain of safety.
Step 1: Ensure Staff Safety with a "No-Touch" Policy
The first and most critical rule is to never touch a fentanyl patch with bare hands. Fentanyl can be absorbed through the skin, and any direct contact is an unnecessary risk.
All staff must use medical gloves when handling returned patches. This simple precaution is the most effective way to prevent accidental exposure.
Step 2: Secure the Patches Immediately
Upon receipt, patches must be safeguarded to prevent diversion. They should not be left on a counter or in any area accessible to the public or unauthorized personnel.
The patches should be placed directly into a secure, temporary holding area or immediately into the final disposal container. This minimizes the window of opportunity for them to be misplaced or stolen.
Step 3: Utilize an Approved Disposal System
Returned fentanyl patches must be placed in a designated, compliant disposal bin for unserviceable narcotics and controlled drugs. These are not standard sharps containers or trash cans.
In jurisdictions like Canada, pre-authorization from regulatory bodies such as Health Canada is no longer required for the local destruction of these substances, streamlining the process for pharmacies.
Why This Protocol is Critical: Understanding the Risks
The strict handling procedures for fentanyl are not bureaucratic formalities; they are essential safeguards against severe and potentially fatal consequences.
The Danger of Residual Medication
A used fentanyl patch is not empty. It can still contain a significant amount of active medication, enough to cause a fatal overdose in someone who is not opioid-tolerant, especially a child.
Folding a used patch so the adhesive sides stick together is a good practice to recommend to patients, as it helps contain the residual drug before it is returned.
Preventing Diversion for Illicit Use
Returned patches are a prime target for diversion. The opioid they contain can be extracted and misused, contributing to the ongoing opioid crisis.
A secure chain of custody within the pharmacy ensures these potent medications are tracked from receipt to destruction, eliminating opportunities for them to enter the illicit market.
Protecting Vulnerable Populations
Improper handling or disposal can lead to tragic accidents. Patches that are not secured can stick to other people, including children or elderly family members, leading to an unintentional and dangerous dose.
Safe take-back programs at pharmacies are the best way to get these patches out of homes where they might pose a risk.
Common Pitfalls to Avoid
Even with a protocol in place, human error can undermine safety. Awareness of common mistakes is key to maintaining a secure system.
Complacency in Handling
The single biggest risk is staff becoming complacent over time. Forgetting to wear gloves or failing to secure a patch immediately can break the chain of safety.
Regular training and reminders about the potency of fentanyl are essential to keep safety top-of-mind for all pharmacy team members.
Inadequate Patient Communication
The pharmacy's role extends to educating patients. Patients must be told never to flush patches down the toilet and to keep them away from children and pets before returning them.
Clear instructions on how to handle used patches at home and the importance of returning them to the pharmacy are a crucial part of a comprehensive safety strategy.
Failure to Document
Proper record-keeping is a legal and professional requirement. Failing to log the return and disposal of a controlled substance can create significant compliance issues for the pharmacy.
Making the Right Choice for Your Pharmacy
Implementing a robust policy for returned fentanyl patches is a non-negotiable aspect of modern pharmacy practice. Use these points to refine your internal procedures.
- If your primary focus is staff and public safety: Your policy must mandate a universal "no-touch" rule using gloves and the immediate isolation of returned patches.
- If your primary focus is regulatory compliance: Ensure you use an approved disposal system and maintain meticulous records for every returned controlled substance.
- If your primary focus is preventing diversion: Your protocol must create a clear and unbroken chain of custody, securing the patches from the moment they are returned until they are rendered non-retrievable.
Your pharmacy is the final, critical line of defense in ensuring these powerful medications are managed safely from prescription to disposal.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Staff Safety | Don medical gloves immediately. | Prevent accidental transdermal exposure to fentanyl. |
| 2. Secure Patches | Log return and isolate patches in a secure area. | Prevent diversion and ensure chain of custody. |
| 3. Proper Disposal | Place in a designated unserviceable narcotics bin. | Ensure compliant and safe destruction of controlled substance. |
Ensure your pharmacy's safety protocols are as reliable as your products.
As a bulk manufacturer of transdermal patches, Enokon understands the critical importance of safe handling from production to disposal. Our expertise in developing reliable, high-quality fentanyl patches and pain plasters is matched by our commitment to safety.
Partner with Enokon for:
- Custom R&D to develop patches that meet precise safety and efficacy standards.
- Technical expertise to support your pharmacy's safe handling and patient education initiatives.
Let's enhance patient and staff safety together. Contact our specialists today to discuss your needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief













