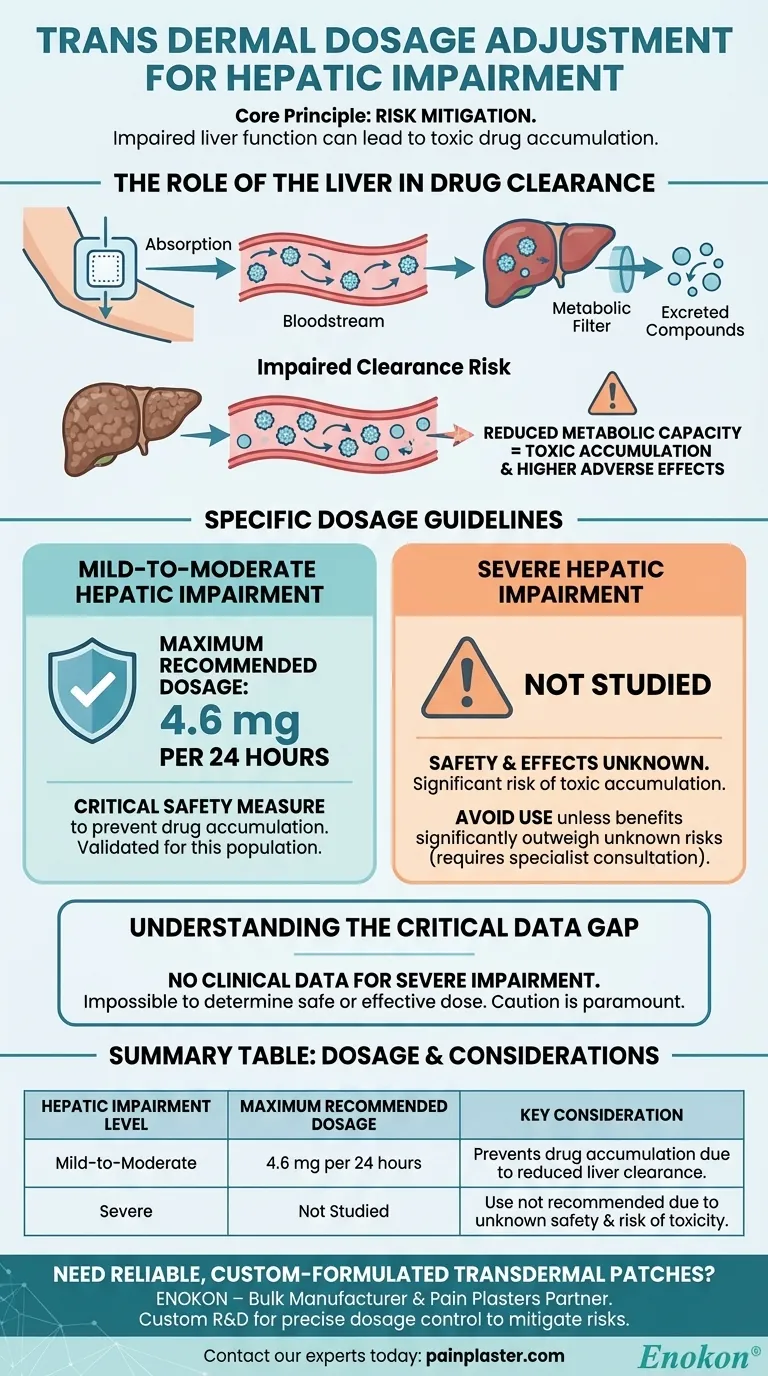
For patients with mild-to-moderate hepatic impairment, the maximum recommended dosage for this transdermal patch should not exceed 4.6 mg per 24 hours. This adjustment is a critical safety measure to account for the liver's reduced ability to process the medication. For individuals with severe hepatic impairment, the safety and effects of this transdermal therapy have not been studied.
The core principle is risk mitigation. Because the liver is responsible for clearing the drug from the bloodstream, impaired liver function can lead to toxic accumulation, even with a transdermal patch. Dose reduction is therefore essential, but this has only been validated for mild to moderate cases.

The Role of the Liver in Drug Clearance
Why Hepatic Function Matters
Even though a drug is absorbed through the skin, it enters the systemic circulation and is eventually transported to the liver. The liver acts as the body's primary metabolic filter, breaking down the drug into compounds that can be easily excreted.
The Risk of Impaired Clearance
When a patient has hepatic impairment, the liver's metabolic capacity is diminished. It cannot clear the drug from the bloodstream at a normal rate.
Avoiding Toxic Accumulation
This reduced clearance means the drug can accumulate in the body over time, leading to higher concentrations than intended. This increases the risk of dose-dependent adverse effects, making a lower dose necessary to maintain a safe therapeutic window.
Specific Dosage Guidelines Based on Impairment Level
Mild-to-Moderate Hepatic Impairment
For this patient population, a clear ceiling has been established. The dosage must be capped at a maximum of 4.6 mg per 24 hours. This is a specific, evidence-based guideline designed to prevent drug accumulation.
Severe Hepatic Impairment
It is crucial to understand that the effects of this transdermal medication have not been studied in patients with severe hepatic impairment. The potential for toxic accumulation is significant, but the precise risk is unknown.
Understanding the Key Limitation
The Critical Data Gap
The absence of data for severe hepatic impairment is the most significant limitation. Without clinical studies, it is impossible to determine a safe or effective dose for these patients.
Caution is Paramount
Prescribing this medication to a patient with severe hepatic impairment introduces an unpredictable level of risk. The potential benefits must be carefully weighed against the unknown potential for harm, typically with specialist consultation.
Making the Right Clinical Decision
Your approach must be guided by the patient's specific level of liver function.
- If your primary focus is treating a patient with mild-to-moderate hepatic impairment: Strictly adhere to the maximum recommended dose of 4.6 mg/24 hours and monitor closely for any adverse reactions.
- If your primary focus is treating a patient with severe hepatic impairment: Recognize that this use case is unstudied and carries unknown risks; this therapy should be avoided unless a specialist determines the potential benefits significantly outweigh these risks.
Ultimately, tailoring dosage to a patient's metabolic capacity is a fundamental component of safe and effective therapy.
Summary Table:
| Hepatic Impairment Level | Maximum Recommended Dosage | Key Consideration |
|---|---|---|
| Mild-to-Moderate | 4.6 mg per 24 hours | Prevents drug accumulation due to reduced liver clearance. |
| Severe | Not Studied | Use is not recommended due to unknown safety and risk of toxicity. |
Need reliable, custom-formulated transdermal patches for specific patient populations like those with hepatic impairment?
At Enokon, we are a bulk manufacturer of high-quality transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to develop solutions that prioritize patient safety. Our technical expertise is available for custom R&D to create patches with precise dosage control, helping you mitigate risks for vulnerable patient groups.
Let's discuss your requirements. Contact our experts today to explore how we can support your product development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- What precautions should be taken before using a menthol patch? Essential Safety Tips for Effective Relief
- How does the addition of Gelatin improve the performance of hydrogel patches? Enhance Adhesion & Delivery Efficiency
- Why is a laboratory-grade pH meter necessary for the safety evaluation of transdermal patches? Ensure Skin Safety.
- What precautions should be taken when applying the fentanyl patch? A Complete Safety Guide to Prevent Overdose
- What side effects were associated with the fentanyl patch? Understanding Common and Serious Risks
- What is the active principle and dosage in the topical ketoprofen patch? Key Benefits & Usage Explained
- How do medical transdermal patches function? Discover How They Maintain Stable Blood Drug Concentrations for Patients
- What is the mechanism of action for diethylene glycol monoethyl ether? Optimizing Transdermal drug Permeation
















