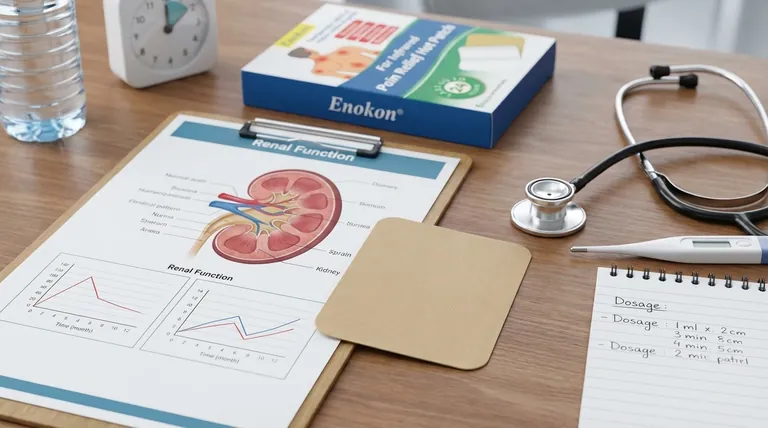
In the absence of formal studies, no specific transdermal dosage adjustments are officially recommended for patients with renal impairment. This lack of guidance, however, signals a need for significant clinical caution, not a confirmation of safety.
The core issue is not that dose adjustments are unnecessary, but that the precise risk is unknown. While transdermal delivery avoids initial liver metabolism, the drug and its byproducts are ultimately cleared by the body's systems, including the kidneys, making organ impairment a critical safety consideration.
Why Organ Function Still Matters with Transdermal Patches
A common misconception is that transdermal patches bypass the risks associated with organ impairment. This is only partially true.
Bypassing the "First-Pass Effect"
When a drug is taken orally, it is absorbed from the gut and passes through the liver before entering the general circulation. This "first-pass effect" can heavily metabolize the drug. A transdermal patch delivers the drug directly into the bloodstream, avoiding this initial process.
The Inevitable Role of Systemic Clearance
However, once the medication is in the bloodstream, it circulates throughout the body. It is eventually metabolized by the liver and excreted by the kidneys, just like drugs administered by other routes. Impaired kidney function can disrupt this final, critical step.
Risk of Drug or Metabolite Accumulation
If the kidneys cannot effectively filter the drug or its active metabolites from the blood, these substances can accumulate. This accumulation can lead to increased side effects or toxicity, even at a standard dose.
A Crucial Comparison: Guidance for Liver Impairment
To understand the significance of the missing renal data, it is helpful to look at the existing guidelines for hepatic (liver) impairment.
Specific Adjustments Are Required
For patients with mild-to-moderate hepatic impairment, a clear dosage adjustment is specified: the transdermal patch dosage should not exceed 4.6 mg every 24 hours.
Severe Impairment Remains Unstudied
Similar to the situation with renal impairment, the effects in patients with severe hepatic impairment have not been formally studied.
What This Comparison Reveals
The existence of a specific dosage cap for moderate liver impairment demonstrates that organ function is a known, critical factor in the safety of this medication. This makes the absence of renal guidelines a significant information gap, highlighting an unquantified risk rather than an absence of risk.
Understanding the Clinical Uncertainty
The primary challenge for clinicians is making a safe decision in the face of incomplete data. The phrase "adjustment may not be required" should be interpreted with extreme care.
The Pitfall of Misinterpretation
It is dangerous to equate "not studied" with "safe to use without adjustment." The true meaning is that no evidence-based recommendation can be made, and the responsibility for patient safety relies on cautious clinical judgment.
The Principle of Cautious Dosing
In pharmacology, when a drug's clearance pathway is compromised and specific data is lacking, the standard of care is to assume that accumulation is possible. Treatment should therefore be initiated at the lowest possible dose with rigorous monitoring.
Making the Right Choice for Your Patient
Given the lack of definitive data, a conservative and patient-centric approach is paramount. All decisions should be based on a careful risk-benefit analysis for the individual.
- If your patient has any degree of renal impairment: Initiate therapy at the lowest available dose and monitor the patient meticulously for any adverse effects, which may signal drug accumulation.
- If weighing the overall risk: Use the established dose limit for mild-to-moderate hepatic impairment (4.6 mg/24h) as an indicator of the drug's sensitivity to organ dysfunction, reinforcing the need for caution.
- If seeking the safest path forward: Consult the full prescribing information for the specific drug and consider a consultation with a clinical pharmacist or nephrologist to help navigate the uncertainty.
Ultimately, navigating a lack of data requires prioritizing patient safety through conservative dosing and vigilant monitoring.
Summary Table:
| Key Consideration | Implication for Renal Impairment |
|---|---|
| Official Guidance | No specific dosage adjustments are formally recommended due to a lack of studies. |
| Actual Risk | Drug/metabolite accumulation is possible as kidneys are a primary clearance pathway. |
| Clinical Approach | Initiate at the lowest possible dose and monitor closely for adverse effects. |
| Reference Point | Use hepatic impairment guidelines (e.g., max 4.6 mg/24h) as an indicator of sensitivity. |
Navigating complex transdermal dosing requires a reliable manufacturing partner.
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise in custom R&D and development ensures your products are formulated with precision and safety in mind, even for sensitive patient populations.
Let us help you develop safe and effective transdermal solutions.
Contact our experts today to discuss your custom patch requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery












