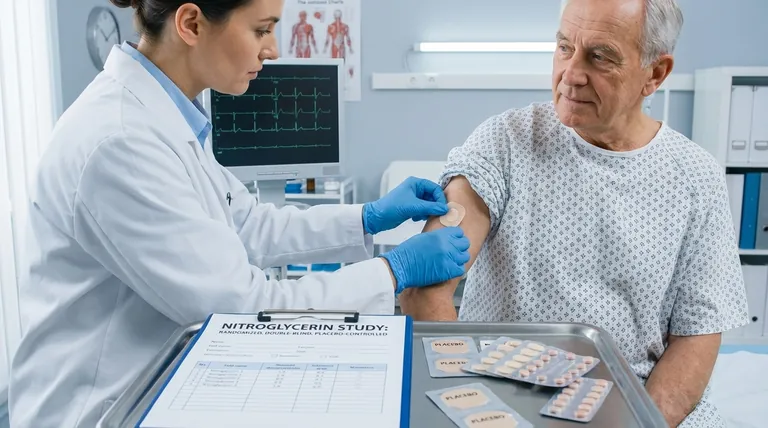
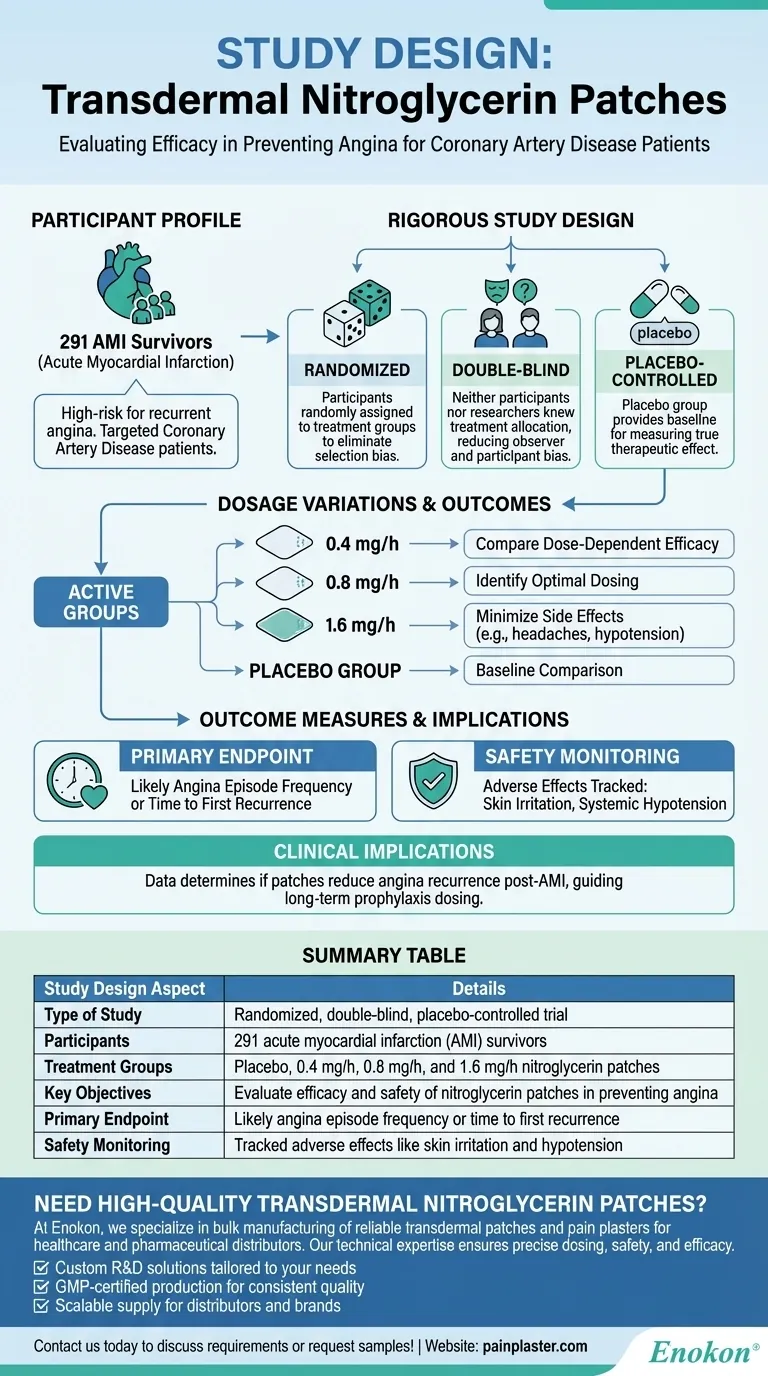
Nitroglycerin transdermal patches are designed to prevent angina episodes in coronary artery disease patients by delivering controlled doses of nitroglycerin through the skin. A key study on these patches was structured as a randomized, double-blind, placebo-controlled trial involving 291 acute myocardial infarction (AMI) survivors. Participants were divided into groups receiving either placebo or active patches with varying nitroglycerin doses (0.4, 0.8, or 1.6 mg/h). This design ensured rigorous evaluation of efficacy and safety while minimizing bias.
Key Points Explained:
1. Study Design: Randomized, Double-Blind, Placebo-Controlled
- Randomization: Patients were randomly assigned to treatment groups to eliminate selection bias and ensure comparable baseline characteristics.
- Double-Blind: Neither participants nor researchers knew who received the active nitro transdermal patch or placebo, reducing observer and participant bias.
- Placebo-Controlled: The placebo group provided a baseline to measure the true therapeutic effect of nitroglycerin patches.
2. Participant Profile
- The study enrolled 291 AMI survivors, a population at high risk for recurrent angina, making the findings clinically relevant for preventive therapy.
- Coronary artery disease patients were targeted, as nitroglycerin’s vasodilatory effects address their narrowed blood vessels and oxygen demand.
3. Dosage Variations
- Patches delivered 0.4, 0.8, or 1.6 mg/h of nitroglycerin, allowing researchers to:
- Compare dose-dependent efficacy.
- Identify optimal dosing for angina prevention while minimizing side effects (e.g., headaches or hypotension).
4. Outcome Measures
- Primary Endpoint: Likely angina episode frequency or time to first recurrence, though specific metrics weren’t detailed in the provided references.
- Safety Monitoring: Adverse effects (e.g., skin irritation or systemic hypotension) were tracked across dosage groups.
5. Clinical Implications
- The design ensured reliable data on whether nitroglycerin patches could reduce angina recurrence post-AMI.
- Findings would guide dosing recommendations for long-term prophylaxis in high-risk patients.
This structured approach highlights how robust trial design can validate the therapeutic role of transdermal nitroglycerin in managing chronic cardiovascular conditions.
Summary Table:
| Study Design Aspect | Details |
|---|---|
| Type of Study | Randomized, double-blind, placebo-controlled trial |
| Participants | 291 acute myocardial infarction (AMI) survivors |
| Treatment Groups | Placebo, 0.4 mg/h, 0.8 mg/h, and 1.6 mg/h nitroglycerin patches |
| Key Objectives | Evaluate efficacy and safety of nitroglycerin patches in preventing angina |
| Primary Endpoint | Likely angina episode frequency or time to first recurrence |
| Safety Monitoring | Tracked adverse effects like skin irritation and hypotension |
Need high-quality transdermal nitroglycerin patches for your patients?
At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors. Our technical expertise ensures precise dosing, safety, and efficacy—ideal for cardiovascular applications.
✅ Custom R&D solutions tailored to your needs
✅ GMP-certified production for consistent quality
✅ Scalable supply for distributors and brands
Contact us today to discuss your requirements or request samples!
Visual Guide
Related Products
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are potential side effects of HRT patches? A Guide to Managing Skin & Systemic Reactions
- What are some tips for applying HRT patches? Ensure Proper Use for Maximum Effectiveness
- What are the benefits of HRT patches? Relieve Menopause Symptoms with Direct, Steady Relief
- What should be done if an HRT patch falls off? Quick Fixes & Prevention Tips
- How often are HRT patches typically applied? A Guide to Once or Twice-Weekly Schedules
















