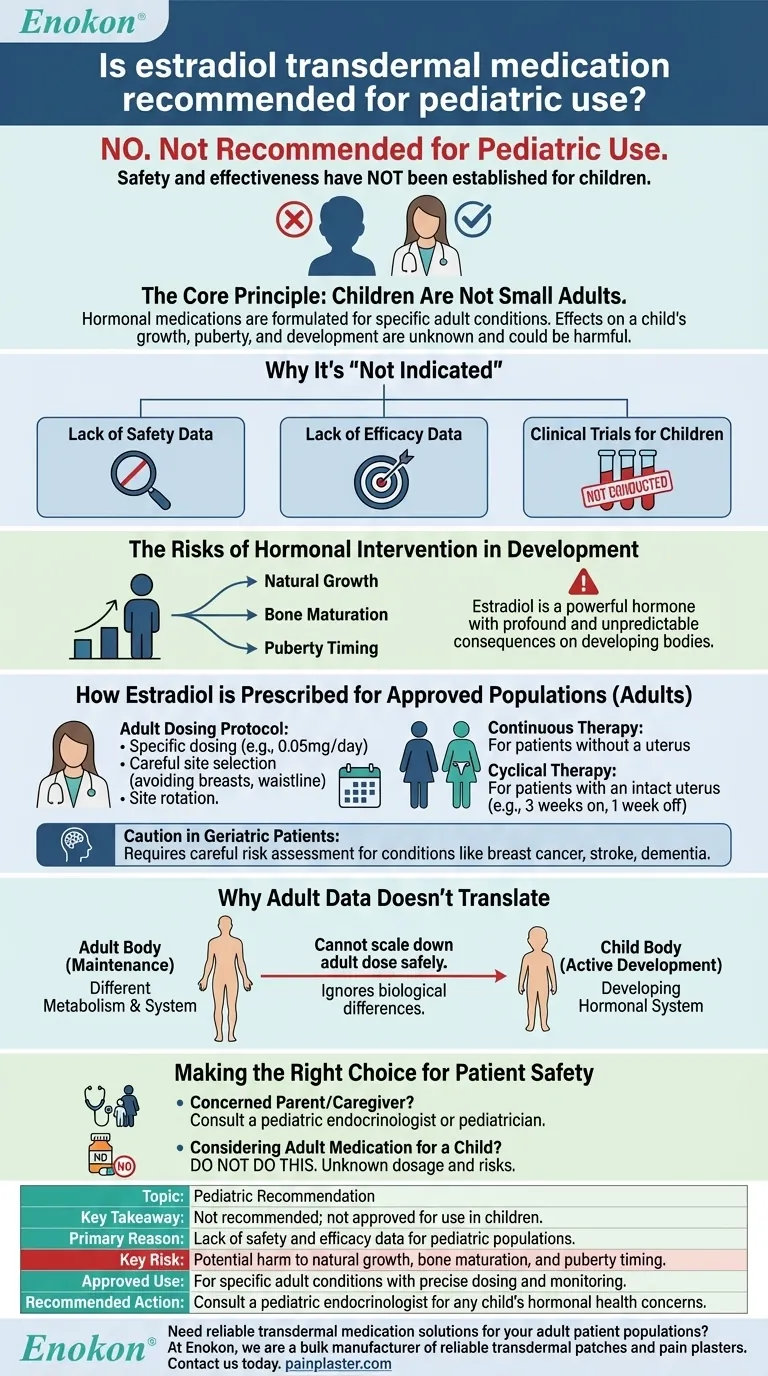
No, estradiol transdermal medication is not recommended for pediatric use. This medication, whether in patch, gel, or spray form, is not indicated for children because its safety and effectiveness have not been established for this population. Introducing potent hormones to a developing body carries unknown risks and is not an approved medical practice.
The core principle is that children are not small adults. Hormonal medications like transdermal estradiol are formulated and tested for specific adult conditions, and their effects on a child's growth, puberty, and overall development are not understood and could be harmful.

The Core Reason: Lack of Safety and Efficacy Data
The decision to not recommend a medication for a specific group is not arbitrary. It is based on a rigorous scientific and regulatory process designed to protect patients, especially vulnerable ones like children.
Why Pediatric Use Is "Not Indicated"
In medical terms, "not indicated" means a drug has not been approved for a particular use or population.
This is because the necessary clinical trials to prove the medication is both safe (does not cause undue harm) and efficacious (actually works for the intended purpose) have not been conducted in children.
The Risks of Hormonal Intervention in Development
Estradiol is a powerful hormone that plays a critical role in sexual development and other bodily functions.
Introducing an external source of this hormone into a child's system could have profound and unpredictable consequences on their natural growth, bone maturation, and the timing of puberty.
How Estradiol is Prescribed for Approved Populations
To understand why this medication is not for children, it is helpful to see how carefully it is managed in the adult populations for which it is approved. This highlights the precision required.
The Adult Dosing Protocol
For adults, treatment is highly specific. A typical starting dose might be a 0.05mg/day patch applied twice weekly.
Application sites are carefully chosen on the trunk, specifically avoiding the breasts and waistline. To prevent skin irritation, the application site must be rotated with each new patch.
Continuous vs. Cyclical Therapy
The administration schedule depends on the patient's specific physiology.
Patients with an intact uterus typically follow a cyclical schedule (e.g., three weeks on, one week off) to manage effects on the uterine lining. Those without a uterus may receive it continuously.
Caution in Geriatric Patients
Even in other tested populations, caution is required. Elderly patients are more likely to have conditions like breast cancer, stroke, or dementia, which require careful risk assessment before and during estradiol therapy.
Understanding the Trade-offs
The core issue is that a drug's effects in one group cannot be assumed to apply to another. The risk-benefit calculation changes dramatically between different patient populations.
The Principle of Indication
Medications are approved for specific indications based on extensive research. Using a drug for a non-approved purpose is known as "off-label" use.
While this sometimes occurs under strict medical supervision, it is exceptionally rare and risky in pediatrics for potent drugs like hormones due to the unknown variables.
Why Adult Data Doesn't Translate
A child's body metabolizes drugs differently than an adult's. Their hormonal systems are in a state of active development, not maintenance.
Simply scaling down an adult dose is not safe or effective. It ignores these fundamental biological differences and can lead to serious adverse effects or therapeutic failure.
Making the Right Choice for Patient Safety
Navigating medical treatments for children requires an unwavering commitment to established safety protocols.
- If you are a parent or caregiver concerned about a child's hormonal health: Your first and only step should be to consult a pediatric endocrinologist or a qualified pediatrician.
- If you are considering using medication intended for an adult on a child: Do not do this. The dosage, absorption rates, and systemic effects are unknown for children and can lead to severe, unintended health consequences.
Always rely on expert medical guidance that is grounded in established pediatric data to ensure a child's health and safety.
Summary Table:
| Topic | Key Takeaway |
|---|---|
| Pediatric Recommendation | Not recommended; not approved for use in children. |
| Primary Reason | Lack of safety and efficacy data for pediatric populations. |
| Key Risk | Potential harm to natural growth, bone maturation, and puberty timing. |
| Approved Use | For specific adult conditions with precise dosing and monitoring. |
| Recommended Action | Consult a pediatric endocrinologist for any child's hormonal health concerns. |
Need reliable transdermal medication solutions for your adult patient populations?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures precise dosing and formulation tailored to your needs. Benefit from our custom R&D and development services to create safe, effective products.
Contact us today to discuss your requirements!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
















