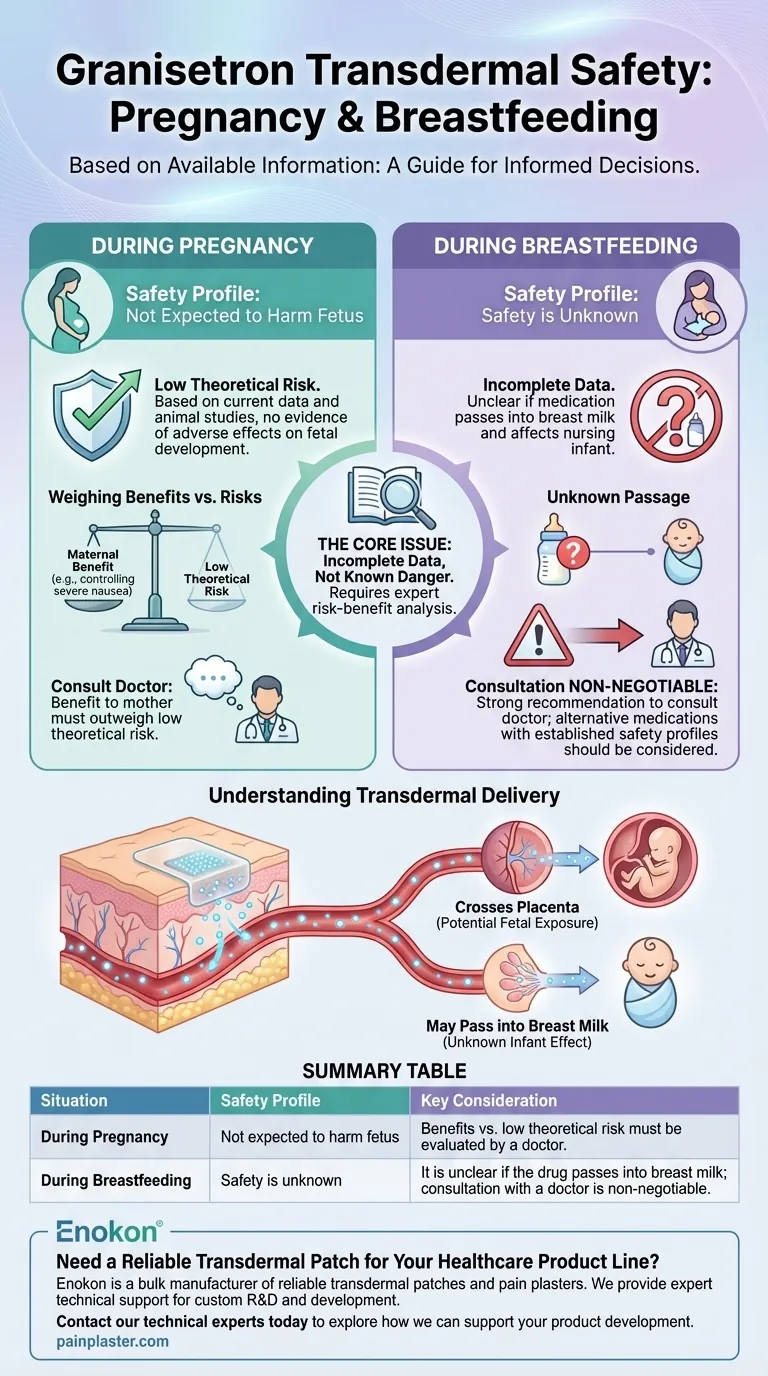
Based on the available information, the granisetron transdermal system is not expected to cause harm to an unborn baby. However, its safety during breastfeeding is unknown because it is not clear if the medication passes into breast milk. Therefore, any use during pregnancy or while nursing requires a direct consultation with your doctor.
The core issue is not one of known danger, but of incomplete data. While the risk during pregnancy appears low, the complete absence of safety information for breastfeeding necessitates a careful risk-benefit analysis with a healthcare provider.

Deconstructing the Safety Profile: Pregnancy vs. Breastfeeding
Understanding the recommendation for granisetron requires separating the distinct considerations for pregnancy from those for breastfeeding. The level of certainty and potential risk differs significantly between the two.
The Outlook During Pregnancy
For pregnancy, the guidance "not expected to harm" suggests that based on how the drug works and any available data (often from animal studies), there is no evidence pointing to adverse effects on a developing fetus.
This classification places it in a category where the benefits of treating the mother's condition (such as severe nausea from chemotherapy) are generally considered to outweigh the low, theoretical risk to the baby.
The Unknowns in Breastfeeding
The primary concern during breastfeeding is whether a drug is excreted into breast milk and, if so, whether it could affect the nursing infant.
For the granisetron transdermal system, this information is simply not available. Without data, it is impossible to establish a safety profile, leading to the strong recommendation to consult a doctor, who may advise against its use while nursing.
Why Transdermal Systems Require Specific Analysis
A transdermal patch delivers medication slowly and continuously into the bloodstream. This means that while it isn't swallowed like a pill, the active drug circulates throughout the body.
Because the drug enters the systemic circulation, it can cross the placenta to the fetus or pass into breast milk. Each transdermal medication is unique; for example, some drugs like oxybutynin have shown no adverse effects in animal studies, while others are known to have potential risks, reinforcing that safety is drug-specific, not delivery-method-specific.
Understanding the Medical Recommendation Framework
When clear human safety data is unavailable—as is common for medications in pregnancy and breastfeeding—doctors rely on a framework of established principles to guide their advice.
The Principle of "Weighing Benefits vs. Risks"
This is the cornerstone of decision-making. Your healthcare provider will evaluate the severity of your medical condition against the potential, often unknown, risks of the medication to your child.
For a mother suffering from debilitating nausea, the benefit of using granisetron to maintain her own health and nutrition may be deemed essential, especially when the fetal risk is considered low.
The Role of Animal Studies
Often, the only available safety data comes from studies conducted on animals. While these studies can identify potential red flags, they do not always translate perfectly to human physiology.
This is why even with no observed adverse effects in animal studies, a medication is rarely declared "completely safe" for human pregnancy.
Why a Doctor's Consultation is Non-Negotiable
General information cannot replace personalized medical advice. A doctor considers your specific health status, the stage of your pregnancy or the age of your nursing infant, and alternative treatment options.
They have the expertise to interpret the limited available data and help you make the most informed choice for your unique situation.
Making an Informed Decision with Your Doctor
Navigating medication choices during pregnancy and breastfeeding is about managing uncertainty. Your goal is to work with your healthcare provider to make a decision that prioritizes the well-being of both you and your child.
- If you are pregnant: Discuss with your doctor whether the benefit of controlling your symptoms with granisetron outweighs the low theoretical risk to the fetus.
- If you are breastfeeding: Ask your doctor to evaluate the unknown risks and strongly consider alternative medications with more established safety profiles for nursing infants.
Ultimately, your healthcare provider is your most critical partner in making a safe and confident decision.
Summary Table:
| Situation | Safety Profile | Key Consideration |
|---|---|---|
| During Pregnancy | Not expected to harm the fetus | Benefits vs. low theoretical risk must be evaluated by a doctor. |
| During Breastfeeding | Safety is unknown | It is unclear if the drug passes into breast milk; consultation with a doctor is non-negotiable. |
Need a Reliable Transdermal Patch for Your Healthcare Product Line?
As a pharmaceutical distributor or brand, you need a manufacturing partner you can trust, especially for sensitive applications. Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters. We provide expert technical support for custom R&D and development, ensuring your products meet the highest standards of safety and efficacy.
Let's discuss your project requirements. Contact our technical experts today to explore how we can support your product development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief












