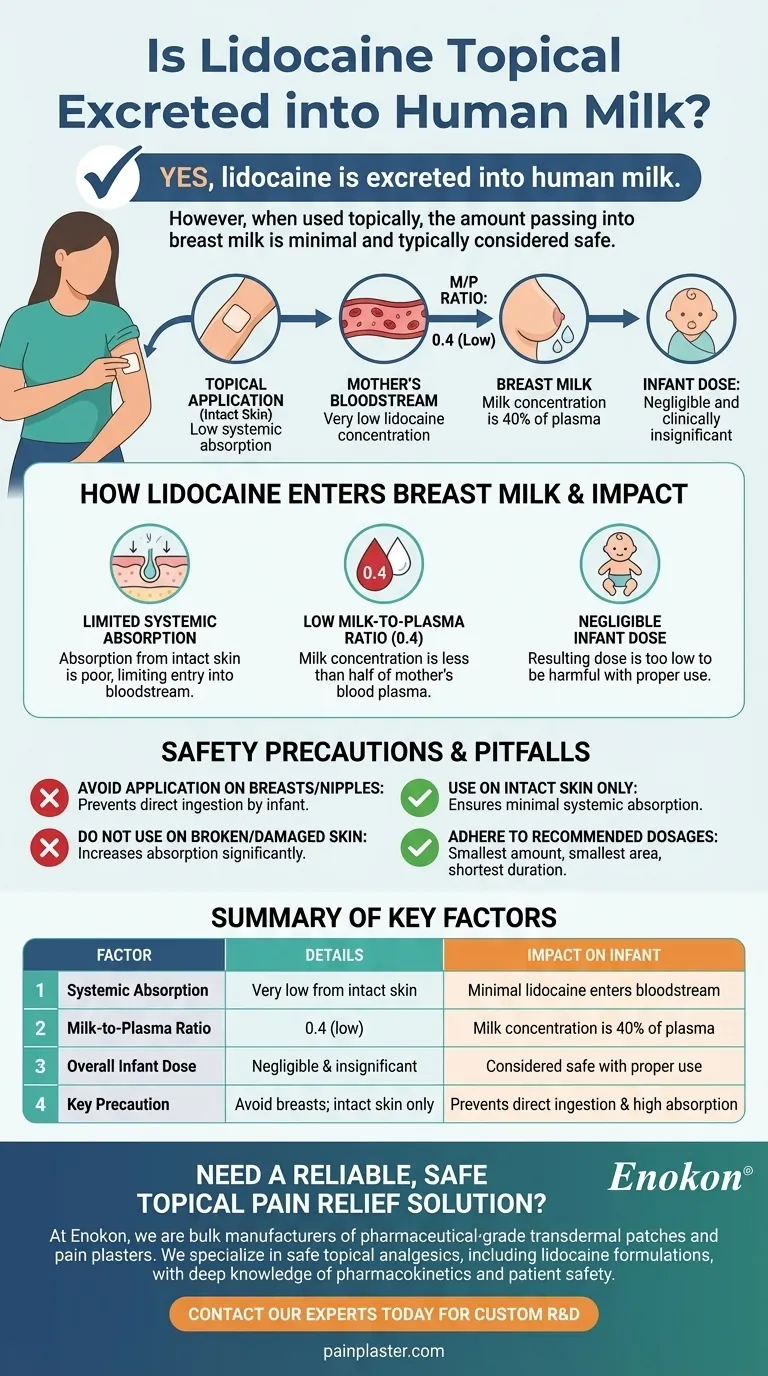
Yes, lidocaine is excreted into human milk. When used topically, the amount that enters the bloodstream is typically very low, and consequently, the amount that passes into breast milk is also minimal. The established milk-to-plasma ratio for lidocaine is 0.4, indicating the concentration in milk is less than half of what is present in the mother's blood.
While topical lidocaine does pass into breast milk, the amount is almost always too low to have any harmful effect on a nursing infant, especially when applied correctly to a small area of intact skin.

How Lidocaine Enters Breast Milk
For any medication to reach breast milk, it must first be absorbed into the mother's bloodstream, a process known as systemic absorption. The way a drug is administered plays a crucial role in how much enters circulation.
The Impact of Topical Application
Topical application, by design, limits a drug's entry into the bloodstream. The goal is to create a local anesthetic effect in the skin, not to distribute the drug throughout the body.
Because systemic absorption from intact skin is poor, the concentration of lidocaine in the mother's blood plasma remains very low.
Understanding the Milk-to-Plasma Ratio
The milk-to-plasma (M/P) ratio is a key measurement for understanding a drug's presence in breast milk.
Lidocaine's M/P ratio is 0.4. This means the concentration of lidocaine in breast milk will only be 40% of the concentration found in the mother's blood plasma. This is considered a low ratio.
The Resulting Infant Dose
The combination of low systemic absorption and a low M/P ratio means the actual dose an infant receives through breast milk is negligible and clinically insignificant.
Common Pitfalls and Safety Precautions
While generally very safe, the risk of systemic absorption can increase if topical lidocaine is not used as intended. Following simple precautions ensures the risk to the infant remains minimal.
Avoid Application on the Breasts
Never apply lidocaine to the nipples or any area of the breast the infant may come into direct contact with during feeding. This prevents direct ingestion of the medication by the baby.
Use Only on Intact Skin
Do not apply lidocaine to broken, inflamed, or burned skin. Damaged skin significantly increases the amount of medication absorbed into the bloodstream.
Adhere to Recommended Dosages
Always use the smallest amount of lidocaine necessary to relieve pain. Apply it to the smallest possible area of skin and for the shortest duration required.
Making a Safe Decision
You can confidently manage localized pain with topical lidocaine while breastfeeding by following proper safety guidelines.
- If your primary focus is treating localized pain (e.g., from stitches or hemorrhoids): Using topical lidocaine as directed on intact skin is considered compatible with breastfeeding.
- If you need to apply it near the breast area: Ensure the skin is thoroughly washed and rinsed of all medication before you begin nursing.
- If you are concerned or need to cover a large area: Always consult with your healthcare provider or a lactation consultant to confirm the best course of action for your specific situation.
Ultimately, making an informed choice based on correct application is the key to using topical lidocaine safely and effectively while breastfeeding.
Summary Table:
| Factor | Details | Impact on Infant |
|---|---|---|
| Systemic Absorption | Very low from intact skin | Minimal lidocaine enters bloodstream |
| Milk-to-Plasma Ratio | 0.4 (low) | Milk concentration is 40% of plasma |
| Overall Infant Dose | Negligible and clinically insignificant | Considered safe with proper use |
| Key Precaution | Avoid application on breasts/nipples; use on intact skin only | Prevents direct ingestion and high absorption |
Need a reliable, safe topical pain relief solution for your healthcare brand?
At Enokon, we are a bulk manufacturer of pharmaceutical-grade transdermal patches and pain plasters. We specialize in developing safe, effective topical analgesics, including lidocaine formulations, with a deep understanding of pharmacokinetics and patient safety profiles—crucial for products used by sensitive populations like breastfeeding mothers.
Let us help you develop a trusted product. Our technical expertise is at your disposal for custom R&D and development.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Heating Pain Relief Patches for Menstrual Cramps
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How long does it take for lidocaine plasters to work? Relief Timeline & Key Insights
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- What should be done if one decides to stop using lidocaine plasters? Safely Transitioning Away from Lidocaine Patches
- How does an industrial-grade ultrasonic processor influence Lidocaine nano-liposomes? Key to Particle Size & Stability
- What are the available dosage forms of topical lidocaine? A Guide to Creams, Gels, Patches & More
- What is the primary function and characteristic of Lidocaine Patches in skin anesthesia? Expert Transdermal Insights
- What types of pain did the lidocaine patch 5% help relieve in the patients? Targeted Relief for Neuropathic and Joint Pain
- What is lidocaine and how is it used in patches? Targeted Pain Relief Explained















