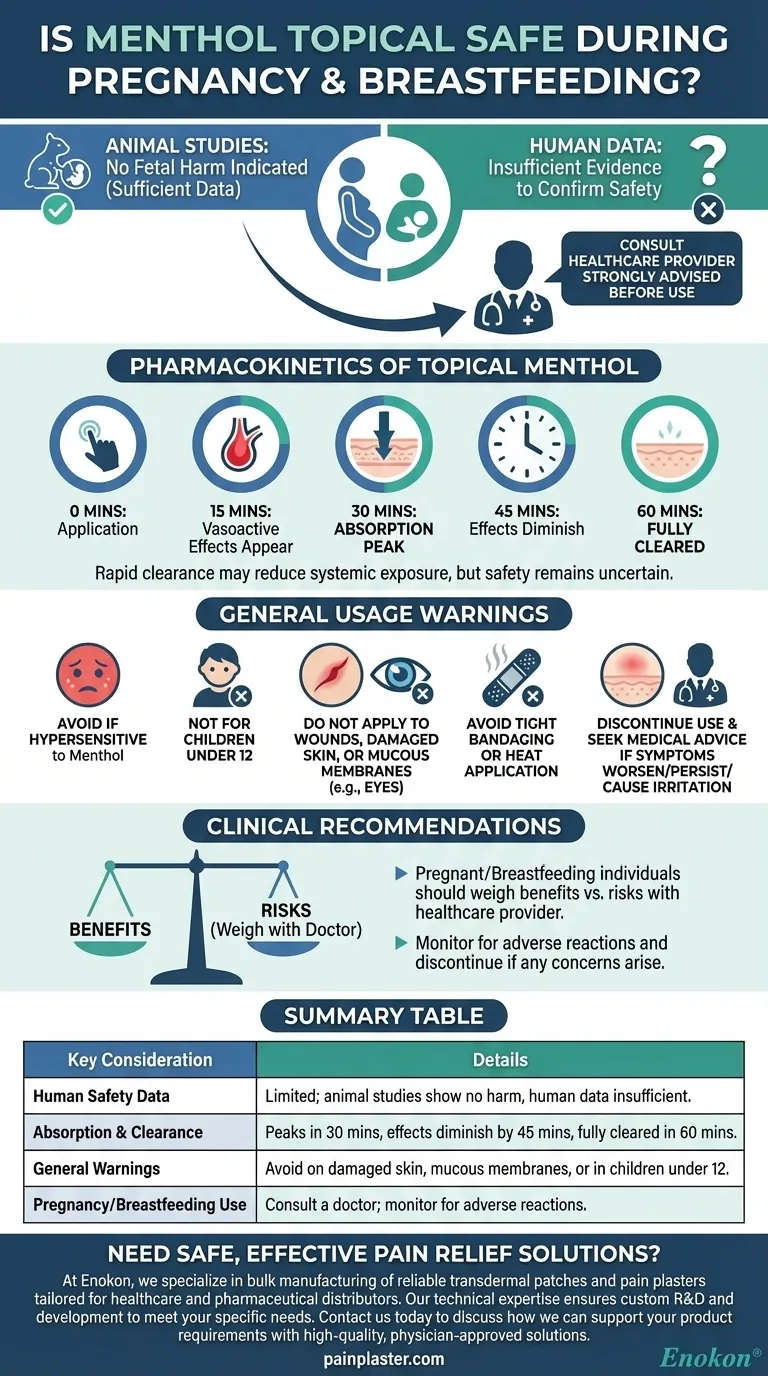
While animal studies indicate no fetal harm from topical menthol, there is insufficient human data to confirm its safety during pregnancy and breastfeeding. The menthol patch is rapidly absorbed and cleared by the skin, but healthcare provider consultation is strongly advised before use. General warnings include avoiding application on damaged skin, mucous membranes, or in children under 12, and discontinuing use if irritation occurs.

Key Points Explained:
-
Limited Human Safety Data
- Animal studies show no fetal harm, but human studies are inadequate.
- Lack of conclusive evidence means risks cannot be fully ruled out.
- Always consult a doctor before use during pregnancy or breastfeeding.
-
Pharmacokinetics of Topical Menthol
- Absorption peaks in the skin within 30 minutes, with vasoactive effects appearing in 15 minutes.
- Effects diminish after 45 minutes, and the drug is fully cleared by 60 minutes.
- This rapid clearance may reduce systemic exposure, but safety remains uncertain.
-
General Usage Warnings
- Avoid if hypersensitive to menthol or other components.
- Not recommended for children under 12.
- Do not apply to wounds, damaged skin, or mucous membranes (e.g., eyes).
- Avoid tight bandaging or heat application after use, as it may increase irritation.
- Discontinue use and seek medical advice if symptoms worsen, persist, or cause redness/irritation.
-
Clinical Recommendations
- Pregnant or breastfeeding individuals should weigh benefits vs. risks with a healthcare provider.
- If used, monitor for adverse reactions and discontinue if any concerns arise.
While menthol patches may offer localized relief, their safety profile in vulnerable populations remains uncertain. A cautious, physician-guided approach is essential.
Summary Table:
| Key Consideration | Details |
|---|---|
| Human Safety Data | Limited; animal studies show no harm, but human data is insufficient. |
| Absorption & Clearance | Peaks in 30 mins, effects diminish by 45 mins, fully cleared in 60 mins. |
| General Warnings | Avoid on damaged skin, mucous membranes, or in children under 12. |
| Pregnancy/Breastfeeding Use | Consult a doctor; monitor for adverse reactions. |
Need safe, effective pain relief solutions? At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters tailored for healthcare and pharmaceutical distributors. Our technical expertise ensures custom R&D and development to meet your specific needs. Contact us today to discuss how we can support your product requirements with high-quality, physician-approved solutions.
Visual Guide
Related Products
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- What is the primary use of a menthol patch? Targeted Relief for Muscle & Joint Pain
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
















