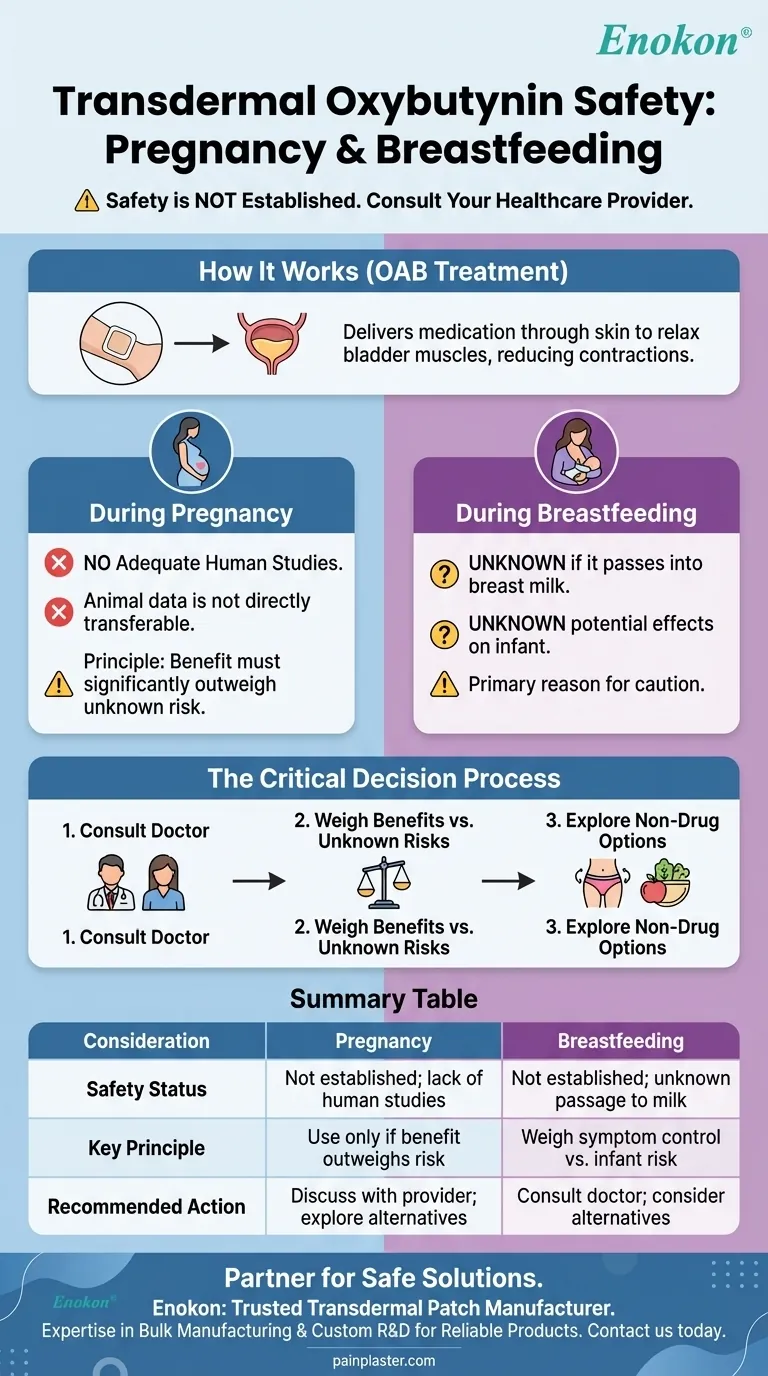
The safety of transdermal oxybutynin during pregnancy and breastfeeding has not been established. Due to a lack of adequate studies in humans, the potential risks to a developing fetus or a nursing infant are not well understood. Any decision to use this medication requires a careful discussion with your healthcare provider.
When managing a condition like overactive bladder during pregnancy or lactation, the core challenge is balancing the mother's need for relief against the unknown risks to the child. The absence of clear safety data for oxybutynin makes a thorough medical consultation essential.

How Transdermal Oxybutynin Works
A Treatment for Overactive Bladder
Transdermal oxybutynin is delivered via a skin patch to treat overactive bladder (OAB). This condition involves uncontrollable contractions of the bladder muscles.
These contractions lead to symptoms like a frequent or urgent need to urinate and an inability to control urination.
Relaxing Bladder Muscles
Oxybutynin belongs to a class of drugs called antimuscarinics. It works by blocking certain nerve impulses, which in turn relaxes the bladder muscles and helps reduce the symptoms of OAB.
The Transdermal Delivery System
The patch is designed to deliver a continuous, steady dose of the medication (typically 3.9 mg per day) through the skin and into the bloodstream. This method can sometimes reduce the side effects associated with oral versions of the drug.
The Critical Gap in Pregnancy Data
Lack of Human Studies
The central issue is the complete absence of adequate and well-controlled studies on oxybutynin use in pregnant women. Without this data, a definitive statement on its safety cannot be made.
Interpreting Animal Studies
While studies on animals have not shown adverse effects at certain doses, this information is not directly transferable to humans. Animal study results do not always predict human outcomes, which is why medical guidance remains cautious.
The Principle of "Clearly Needed"
In situations with limited data, healthcare providers often recommend using a medication only if its potential benefit to the mother significantly outweighs the unknown potential risk to the fetus. This principle is applied to many medications during pregnancy.
Understanding the Risks During Breastfeeding
Unknown Presence in Breast Milk
It is currently unknown if oxybutynin from the transdermal patch passes into human breast milk. Because the drug is absorbed into the mother's bloodstream, it is plausible that it could be.
Potential Effects on the Infant
If the drug were present in breast milk, its potential effects on a nursing infant are also completely unknown. This uncertainty is the primary reason for caution.
The Importance of Medical Consultation
This lack of information makes it critical to discuss your specific situation with a doctor. They can help you weigh the benefits of managing your OAB symptoms against the theoretical risks of exposing your baby to the medication.
Making the Safest Choice for You and Your Baby
Your decision requires a careful, personalized assessment with your healthcare provider, who can evaluate your specific health needs.
- If you are pregnant or planning to become pregnant: Your first step is to discuss the necessity of OAB treatment and explore all potential alternatives, including non-pharmacological options, before considering oxybutynin.
- If you are currently breastfeeding: You and your doctor must weigh the benefits of symptom control against the unknown risks of exposing your infant to the medication through your breast milk.
- If you are exploring non-drug options: Pelvic floor exercises, bladder training, and dietary modifications are often recommended first-line treatments for OAB and carry no risk during pregnancy or lactation.
Ultimately, the safest path is a proactive partnership with your doctor to navigate your treatment options based on your individual circumstances.
Summary Table:
| Consideration | Pregnancy | Breastfeeding |
|---|---|---|
| Safety Status | Not established; lack of human studies | Not established; unknown if it passes into breast milk |
| Key Principle | Use only if benefit to mother outweighs potential risk to fetus | Weigh symptom control benefits against unknown infant risks |
| Recommended Action | Discuss with healthcare provider; explore non-drug options first | Consult a doctor before use; consider alternatives |
Navigating medication safety during pregnancy and breastfeeding requires expert guidance and reliable product quality. If you are a healthcare or pharmaceutical distributor or brand seeking a trusted manufacturing partner for transdermal patches, Enokon is here to support you. We specialize in the bulk manufacturing of reliable transdermal patches and pain plasters. Our technical expertise ensures high-quality, consistent products, and we offer custom R&D and development services to meet your specific needs. Contact us today to discuss how we can help you deliver safe and effective solutions to your customers. Get in touch with our experts
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- Why is a 96-well plate microplate reader necessary for evaluating the cytotoxicity of transdermal drug delivery additives?
- How do older Chinese medicated plasters differ from newer ones? Evolution in Comfort & Efficacy
- What are the potential side effects of buprenorphine patches? Risks & Safety Tips
- What are the dual functions of glycerin within a hydrogel matrix for transdermal delivery? Unlock Optimal Patch Performance
- What is the function of PSA in transdermal drug delivery? Optimize Release and Adhesion for Maximum Efficacy
- How does FTIR assist in transdermal patch development? Ensure Chemical Stability and Material Compatibility
- What are the guidelines for applying pain relief patches? A Step-by-Step Guide for Safe & Effective Use
- Why is a site rotation strategy necessary for the application of Asenapine transdermal patches? Boost Safety & Efficacy


















